SPRING 2020, THE EVIDENCE FORUM, WHITE PAPER
 Mariah Baltezegar, MBA Head of Peri- and Post-Approval Virtual Trials Real-World Evidence Evidera |  Teresa Wilcox, PhD Vice President Real-World EvidenceEvidera |
Multiple strategies, each with their individual advantages and drawbacks, may be employed to answer a research question, but determining the optimal strategy – the one that will bring you to your answer in the most effective and efficient way possible – that is the true challenge in our industry. Identifying that optimal strategy requires the synthesis of science, operations, and increasingly, technology. That synthesis, with its balance and integration of so many elements, cannot be achieved with a scattershot approach. Our systematic approach allows us to consider all these elements, to create that required synthesis, and to find that optimal strategy for each research question. This comprehensive process engages and considers the needs of cross-functional and multi-stakeholder subject matter experts (SMEs), including internal client stakeholders, study design experts, healthcare providers, external partners, other potential stakeholders, and most importantly, patients and their caregivers.
Our approach focuses on three key steps, including a two-step feasibility assessment and then a strategy confirmation.
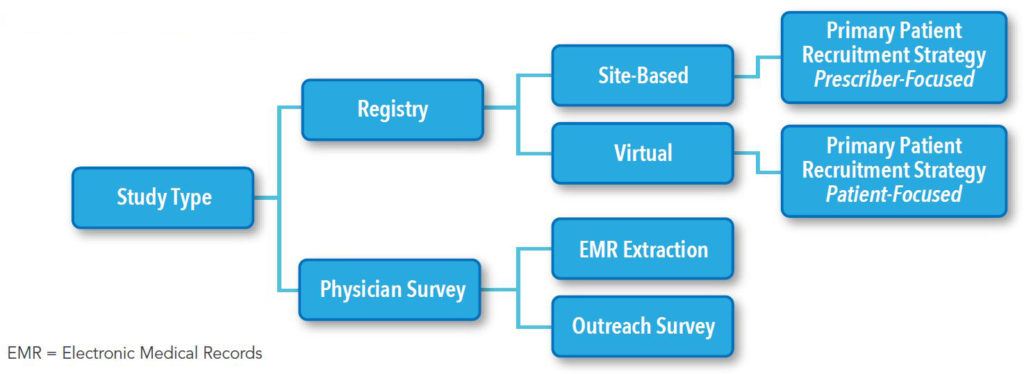
LEVEL ONE FEASIBILITY: An assessment of possible strategies to answer the research question(s) of interest, including:
- Detailed and specific scientific considerations and questions
- Review of what is vital to the return on investment (ROI) assessment (e.g., regulatory requirements, critical assessments, length of data collection, patient retention, budget constraints)
- Assessment of the optimal data collection method
LEVEL TWO FEASIBILITY: Refinement of strategy informed by stakeholder feedback, including:
- Analysis of study design options
- Assessment of execution options
STRATEGY CONFIRMATION: Specific recommendations
The two-step feasibility assessment considers the benefits of digital enablement at each point of the evidence generation process. While technology enablement uses a tool to produce an outcome, digital enablement is choosing the right technology to elevate and advance, in this case, evidence generation.
Level One Feasibility
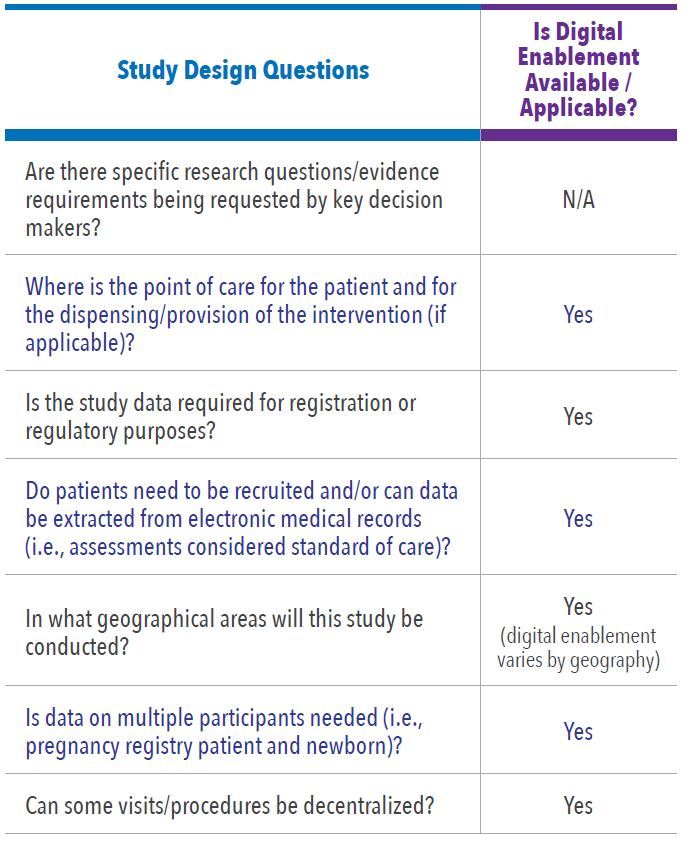
Assessment of Approach to Answer Research Questions
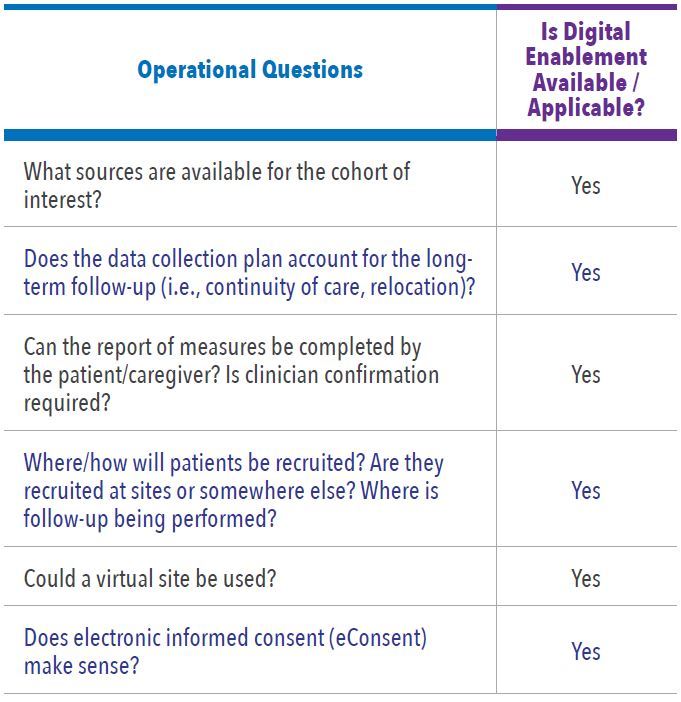
In Level One Feasibility there are key questions and possible frameworks to be considered for each study. Figure 1 illustrates the flow of how we assess the study design process. Table 1 and Table 2 list the examples of the key questions for assessing study design and operations options, and whether digital enablement can contribute.
Level Two Feasibility
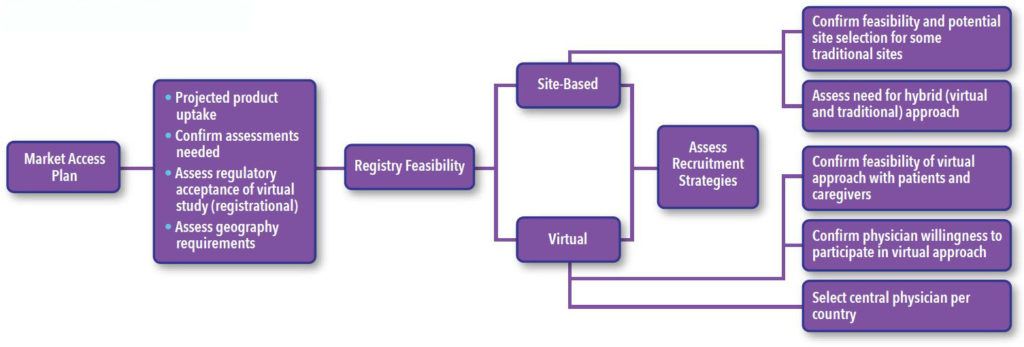
Refinement of Strategy Informed by Stakeholder Feedback
In Level Two Feasibility our study approach is assessed based on key features of each study and considerations based on answers received from the Level One Feasibility, ultimately informing the recommended approach. The next step is scientific and operational assessment of a series of questions and factors that inform the final design and operational strategy (See Figure 2).
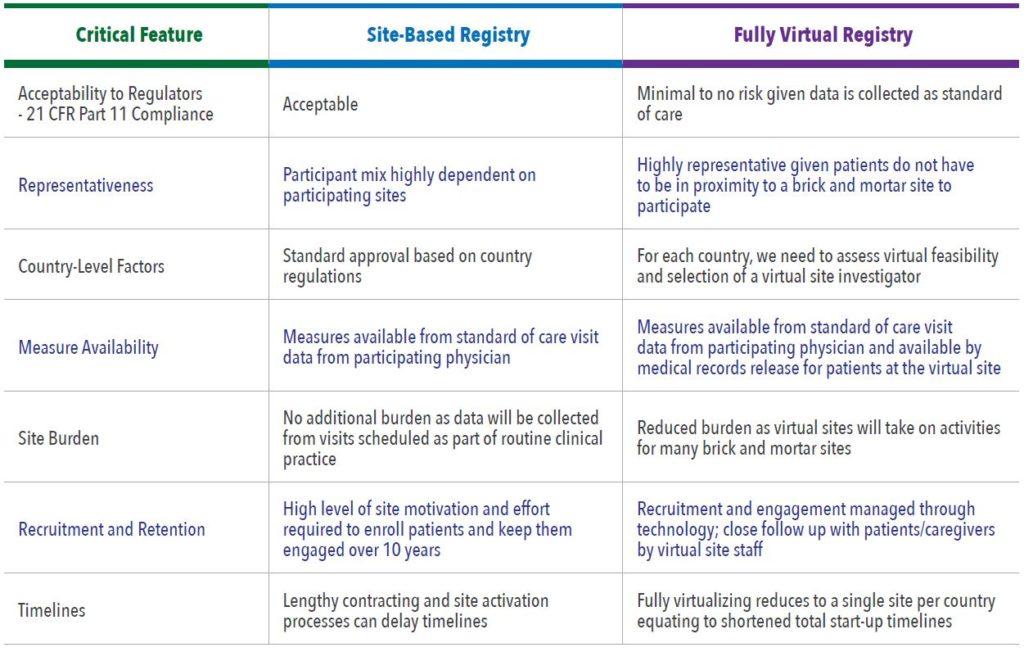
Critical Features to Assess
The following critical areas must be assessed when determining the best study design to answer a research question:
- Acceptability to Regulators, including 21 CFR Part 11 Compliance1
- Alignment to the European Union’s General Data Protection Regulation (GDPR)2
- Representativeness
- Country-Level Factors
- Availability of Measures
- Appropriate Data Reporter (Clinician, Patient)
- Patient/Caregiver Burden
- Site Burden
- Recruitment and Retention
- Timelines
- Cost
- Challenges and Opportunities
Considering Decision Maker Needs in Solution Development
Example: Regulatory Guidance Regarding Use of RWE to Address PASS
Should the key audience for the study findings be a regulator(s), the evidence generation approach must consider their requirements.
- The European Medicines Agency’s guideline on good pharmacovigilance practices (GVP)3 acknowledges real-world evidence (RWE) approaches for post-authorization safety studies (PASS) for both primary and secondary data. Most PASS are observational studies and increasingly introduce other objectives, such as real-world utilization (in particular, to describe exposure in groups that have not been exposed in clinical trials) and effectiveness outcomes, on top of safety outcomes, drug utilization (42%), and effectiveness (30%).
- In the US, the use of RWE for regulatory decision making was mandated in the 21st Century Cures Act1 of December 2016, and a framework for its incorporation into decisions is provided in the Framework for FDA’s Real-World Evidence Program4 of December 2018. Although additional regulatory guidance documents are under development, the Cures Act and FDA RWE Framework provide sponsors with an array of study design options for the post-approval setting.
- Beyond Europe and the US, which have been followed closely by Canada and Australia, Asian countries such as South Korea, India, Japan, and mainland China also now request post-marketing, real-world evidence to observe drug effects both in routine practice conditions and in larger and more diverse populations.
Engagement of the Patient in Solution Development
The 21st Century Cures Act has expanded the focus on patient centricity by introducing “Patient-Focused Drug Development” and developing a plan to issue guidance on how to include the patient experience in drug development and regulatory decision making. The inclusion of patient centricity in drug development can involve a multitude of activities. One aspect is the use of patient-reported outcomes (PROs) to collect patient experiences; however, this remains infrequent with a recent study showing that only six out of 30 registries collected data on measures of quality of life.
A virtual approach to study execution potentially reduces timelines and cost, enabling patients and caregivers across the globe to participate in a study while minimizing burden. Through the virtual model, the emphasis on sites and in-person monitoring can be foregone for remote data collection and electronic communication between physician and patient. This provides the researchers, patients, caregivers, and other stakeholders and data providers the flexibility to ease trials into their everyday work and life.
Case Study Example
Overview of Study Design
In this hypothetical case study, we outline the above approach for a study with these characteristics:
- A non-interventional registry study
- Evaluating long-term safety and effectiveness of a medication as used in routine clinical practice in adult patients (18 years of age or older)
- Designed specifically to meet a post-approval safety commitment
Strategy Recommendations
For this study, we recommend a virtual approach to collect study data outlined. This approach provides:
- Involvement of fewer countries and sites
- Reduced burden of participation for sites and patients
- Long-term engagement and retention of sites and patients
Other designs discussed in Level One Feasibility: Assessment of Approach to Answer Research Questions were considered. While database analytics is acceptable to regulatory bodies for assessment of long-term safety risks, other key decision makers may be interested in comparative treatment effectiveness, which is not feasible using data analytics alone since current databases rarely have continuity over extended time periods. The clinician and patient assessments of treatment effect are rarely recorded in the medical record, neither as defined fields nor in text fields.
Virtual Model
Given the procedures are all standard of care in this case study example, we proposed conducting the study as a fully virtual study. This provides:
- Reduced country footprint
- Reduced site footprint
- Reduced patient, caregiver, and healthcare provider burden
- Increased potential patient pool given a patient’s ability to participate from anywhere
- Increased patient engagement through digital enablement of engagement strategies and data collection
Conclusion
Real-world evidence research questions can be answered in a myriad of ways. Each solution has benefits and risks that must be weighed. Whether it is the quality or quantity of the data or cost of the procurement of the data, these risks and benefits need to be methodically assessed. This assessment must include scientific study design questions, operational execution questions, and align patient centricity and digital enablement. When assessment is underpinned by experience and strong capabilities, the resulting solution ensures a well-thought out, key stakeholder engaged approach.

References
- US Food and Drug Administration (FDA). CFR–Code of Federal Regulations Title 21, Part 11. Electronic Code of Federal Regulations. Available at: https://www.ecfr.gov/cgi-bin/text-idx?SID=4e42fd4abdcac4c51b8936bd1681e273&mc=true&tpl=/ecfrbrowse/Title21/21cfr11_main_02.tpl. Accessed March 18, 2020.
- GDPR.EU. General Data Protection Regulation. Complete Guide to GDPR Compliance. Available at: https://gdpr.eu/. Accessed March 18, 2020.
- European Medicines Agency (EMA). Good Pharmacovigilance Practices. Available at: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices. Accessed March 18, 2020.
- US Food and Drug Administration (FDA). Framework for FDA’s Real-World Evidence Program. Available at: https://www.fda.gov/media/120060/download. Accessed March 18, 2020.
For more information, please contact us.