FALL 2019, THE EVIDENCE FORUM, WHITE PAPER
 Carla Dias-Barbosa, MSc Research Scientist Patient-Centered Research Evidera |  Asha Hareendran, PhD Senior Research Leader Patient-Centered Research Evidera |
Context and Background
Emerging trends for ensuring patient centricity in healthcare decisions by regulators and payers challenge the traditional evidence hierarchy where quantitative research-based knowledge was the strongest evidence, with clinicians’ evaluation of outcomes taking precedence over patient reports of their experiences and opinions.1
Healthcare decision making systems use health technology assessment (HTA) to inform the reimbursement decisions for new technologies. Health technology assessment is defined as “the systematic evaluation of the properties and effects of a health technology, addressing the direct and intended effects of this technology, as well as its indirect and unintended consequences, and aimed mainly at informing decision making regarding health technologies. HTA is conducted by interdisciplinary groups that use explicit analytical frameworks drawing on a variety of methods.2”
Appraisals of value conducted by HTA agencies vary in terms of stakeholder involvement, methodology, and processes used, including the evidence base considered and how the results are presented and communicated.3 Value may be considered in terms of clinical, economic, and patient-relevant outcome improvements, often in the context of societal and ethical considerations.
There is growing emphasis on the need for more patient-centered methods in the development and evaluation of new technologies. A recent stakeholder survey showed that there was a clear consensus across health technology, industry, and patient representative stakeholders on the importance of promoting patient involvement in HTA at a higher level than currently used, however, there is a need for a more structured process and guidance for patient involvement.4 While patients are increasingly involved in a range of HTA processes, the findings of another survey conducted among fifteen HTA bodies from twelve countries revealed that only a few HTA organizations evaluate their patient involvement activities.5 Furthermore, there was some question regarding what constitutes a “meaningful” patient engagement and how it might be assessed, suggesting a desire to move away from less meaningful practices and a need to ensure that the patient involvement approaches taken add value to the process and to the parties involved.6
Several HTA agencies and academics associated with HTA are now considering effective ways to incorporate the patients’ or, in some cases more generally, the public’s perspectives in their methods. The involvement of patients in HTA has been conceptualized in terms of:
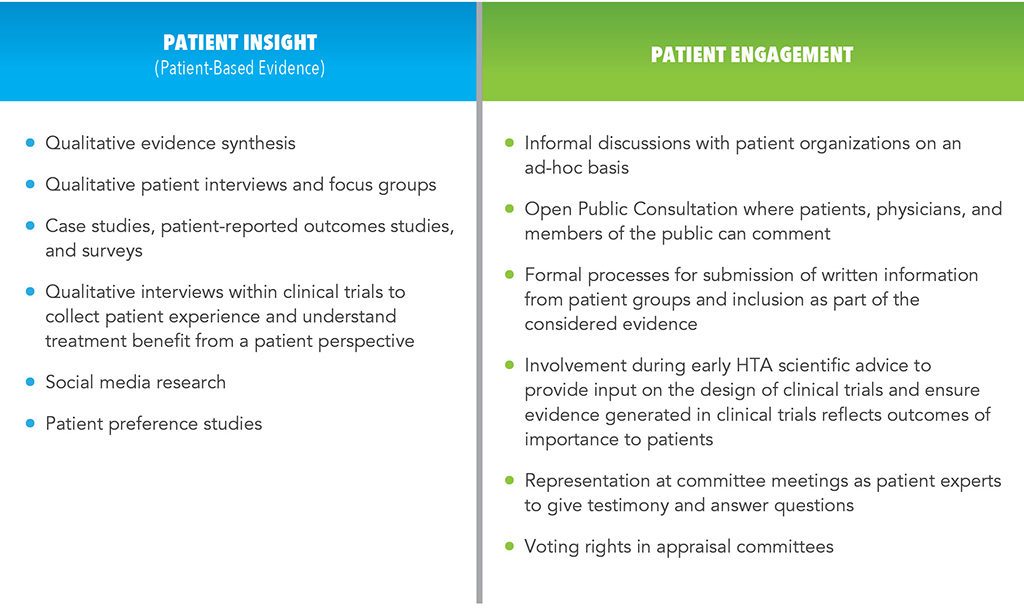
- Consideration of patient insight (also called patient-based evidence [PBE]) collected through research for evaluating health technologies (e.g., patient experience of symptoms and impacts, perceptions of treatment benefits and risks, expectations, preferences). Patient-based evidence can be produced using qualitative and quantitative primary research, and/or performing secondary research that includes published literature on social and ethical issues.
- Patient engagement in the HTA process, potentially from horizon scanning and early consultations for scientific advice through developing recommendations for evaluating health technologies as individuals or as representatives of associations.
A range of methods and opportunities exist to enhance the patient centricity of appraisals of new technologies (See Table 1). Careful consideration and leveraging of these opportunities throughout the drug and device development continuum can contribute to patient centricity of HTA appraisals to ensure the patient voice is heard when determining access to technologies with benefits for patients.
Various stakeholders are working individually and in consort to develop frameworks and tools to enable patient involvement.7-10 These initiatives aim to help prepare, engage, and sustain key stakeholders (e.g., patients, assessors, healthcare decision makers) on the inclusion of patient centricity in methods, processes, and communication of HTA appraisal results.
Opportunities to Enhance Patient Centricity of HTA Appraisals
Regulatory agencies, such as the US Food and Drug Administration (FDA), have pushed the patients’ voice into the center of drug development and regulatory decisions by launching programs such as the Patient-Focused Drug Development (PFDD) initiative11 that aims to ensure patients’ experiences, perspectives, needs, and priorities are captured and meaningfully incorporated in drug development and evaluation. The FDA also led efforts to provide guidance about the methods to be used for developing tools to support label claims and for interpreting data based on patient-reported outcome (PRO) measures.12 More recently the FDA has been open to considering evidence based on qualitative research to ensure the patient perspectives on the value of treatment can be adequately captured using scientific methods.13
Although the general trend is toward an increased consideration of patient insight in HTA, recent reviews have shown limited examples that illustrate the use of PBE in HTA submissions. A systematic review of HTA submissions (with decisions published after January 1, 2012) to 12 HTA bodies in 7 chronic diseases showed that factors related to patient experience (route of administration, disease burden, impact on caregivers) were only discussed in 11% of HTA appraisals (19/168).14
… factors related to patient experience (route of administration, disease burden, impact on caregivers) were only discussed in 11% of HTA appraisals …
While some HTA assessors tend to consider PBE lower in the evidence hierarchy and have limited impetus to integrate this type of evidence in the evaluation process, a few exceptions do exist as in the examples below.15
- Several HTAs produced by the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) incorporated information based on qualitative evidence synthesis of patients’ experiences. For example, for the HTA of intervention programs for self-harming, the SBU conducted a systematic literature review of qualitative research studies to understand the experiences and perceptions of people who self-harm with reference to healthcare and school personnel.16
- The Scottish Health Technologies Group’s (SHTG) HTA of antimicrobial wound dressings in patients with chronic leg ulcers used information about patients’ experiences from a literature review, focus groups, and interviews with people in Scotland to formulate conclusions and develop relevant advice. A comprehensive “patient aspects section” based on PBE was developed for the HTA report and this body of evidence was also used to create a patient version of the HTA report.17
- The Canadian Agency for Drugs and Technologies in Health (CADTH) used qualitative evidence synthesis for the assessment of interventions for the treatment of obstructive sleep apnea (OSA). This synthesis considered the perspectives and experiences of patients, their family members, and nonmedical caregivers and contributed to the HTA in three major ways: understanding the clinical findings, informing the recommendation generated by the expert committee, and identification of implementation considerations.18
- An HTA of tumor necrosis factor (TNF)-alpha inhibitors by the National Institute for Health and Care Excellence (NICE) used evidence from a case study and online survey results provided by a patient group to draft and adjust their recommendations.19
There are indeed opportunities for enhancing patient centricity in HTA appraisals through effective patient engagement, increased efficiency of evidence generation and submission, revision of HTA appraisal methods and processes, and effective communication and reporting of HTA appraisals in a manner that is meaningful to all stakeholders.
Effective Patient Engagement in HTA Process
 The role of patient representatives has become critical in drug development and HTA appraisals, specifically during early dialogues with regulators and HTA bodies. A review of patient participation in scientific advice procedures since 2007 shows that in nearly every case (93%) patient input provided added value to scientific advice,20 thus enhancing the need for creating better informed patients.
The role of patient representatives has become critical in drug development and HTA appraisals, specifically during early dialogues with regulators and HTA bodies. A review of patient participation in scientific advice procedures since 2007 shows that in nearly every case (93%) patient input provided added value to scientific advice,20 thus enhancing the need for creating better informed patients.
Resources and education materials exist for patients to understand HTA21-23 as well as programs to assist patient organizations in setting up patient expert advisory boards, or community advisory boards (CABs), and creating informed patients through education and training to enhance their credibility, legitimacy, and power.24
Patient engagement can be particularly valuable in discussions to achieve consensus about relevant outcomes that should be measured and reported in clinical research for evaluation of new technologies. For example, the COMET (Core Outcome Measures in Effectiveness Trials) initiative25 brings together different stakeholders (including patients/patient advocates, clinicians, researchers, HTA representatives, payers, regulators, and research funders) for the development of agreed upon, standardized sets of outcomes, known as “core outcome sets” (COS) to ensure drug development focuses on outcomes of relevance to patients, as well as HTA bodies for informed decision making.
Increased Efficiency of Evidence Generation and Submission
Drug manufacturers can play a role in improving the quality and scientific rigour of PBE submitted as part of their HTA submissions, including using PRO tools that measure outcomes that are relevant to patients, and providing clear rationale to show the meaningfulness of results on PRO-based endpoints and which change scores on these tools translate into meaningful benefits and acceptable risks to patients. The use of consistent relevant outcomes, such as the COS discussed earlier, and methods would also improve the ease of understanding and acceptability of PBE.
Additionally, some HTA bodies (e.g., Scottish Medicines Consortium [SMC], NICE, and CADTH) encourage written submissions from patient groups to capture their input about experiences and expectations of new technologies. To share good practices, the HTAi Interest Group for Patient and Citizen Involvement in HTA published Patient Group Submission Templates for HTA26 and provided guidance about the form and type of information that would be useful for an HTA committee.
Using Methods and Processes that Enable Patient Centricity
New tools and methodological frameworks are being created that can be used at various stages of drug development to influence and enhance HTAs, including:
- The methodological framework developed by EUneHTA (HTA Core Model®)27 for evaluating new technologies and promoting good practices in HTA methods and processes
- The guidance paper for patient involvement in HTA issued by the European Patients’ Academy (EUPATI),21 which lists suggested patient involvement activities for individual HTAs, including:
- identifying and prioritizing health technology for assessment
- scoping (developing a framework for an individual HTA)
- assessing and developing recommendations/guidelines reviewing and disseminating HTA outcomes8,21
Initiatives also focus on the use of patient preference information (PPI) in HTA. The use of PPI in HTA has been relatively limited to date – only two European countries (Germany and Sweden) formally acknowledge the role of PPI in their methods guide and there are examples of PPI being used elsewhere (e.g., England and Wales). However, agencies in various countries (Denmark, England and Wales, Ireland, and the Netherlands) have initiated pilots on the use of PPI, and IMI PREFER28 is investigating the use of PPI in decision making. More detail on the use of PPI can be found in “Patient Preferences in Health Technology Assessment in Europe: Recent Advances and Future Potential” within this issue of The Evidence Forum.
Sustaining Patient Centricity
 Communicating the results of HTA appraisal to patients through user-friendly summaries and proactively providing them with feedback about the value of their contribution is essential for improving patient involvement approaches, but also to sustain their engagement in research. For instance, qualitative evidence synthesis of PBE used in HTA appraisals can guide the creation of patient versions of the HTA reports (as seen in the earlier example on antimicrobial wound dressings17) and support dissemination of HTA results among patient communities.
Communicating the results of HTA appraisal to patients through user-friendly summaries and proactively providing them with feedback about the value of their contribution is essential for improving patient involvement approaches, but also to sustain their engagement in research. For instance, qualitative evidence synthesis of PBE used in HTA appraisals can guide the creation of patient versions of the HTA reports (as seen in the earlier example on antimicrobial wound dressings17) and support dissemination of HTA results among patient communities.
The upcoming European Clinical Trial Regulation29 makes the provision of plain language summaries mandatory for all sponsors conducting interventional clinical trials in the European Union. Under the new regulation, the European Commission will establish a publicly accessible EU database to grant public access to relevant information on clinical trials, including plain language summaries of clinical trial results.
There is an opportunity to ensure that patient centricity in HTA is built into the context of a sustainable partnership with patients with an ethos of respect, sharing, and learning from each other. A successful implementation of this philosophy that could be used as a model is the British Medical Journal (BMJ) which introduced editorial changes aimed at making patient partnership integral to the way the journal works and thinks. Additionally, the journal established patient review of all relevant research papers alongside the standard scientific peer review processes. Such initiatives can promote willingness of both patients and the public to participate and engage in research not only as trial participants but as active partners, while also helping to sustain their engagement.
Conclusion and Future Directions
There is an increasing emphasis on providing patient-centered healthcare and ensuring patient involvement in the development and evaluation of new technologies, and several initiatives and examples of successful patient involvement in drug development and HTA currently exist. However, despite growing efforts for patient involvement in HTA around the globe, there is a need for standardization of methods for running patient and public consultation, managing interactions between different stakeholders, developing structured and efficient frameworks, common tools, and best practices across HTA bodies.
In Europe, the European commission proposed a framework for establishing European HTA collaboration and conducting joint clinical assessments (JCAs) at the EU level.8 Patient involvement is referenced in the JCAs, however, there is a dearth of detail about how such involvement will be operationalized and incorporated in JCAs – and more broadly – in EU HTA. The EU commission proposal for EU HTA offers an exciting opportunity for cross-border cooperation and development and implementation of a common framework for patient involvement in HTA in Europe. Two pivotal areas of patient involvement should be prioritized:
- Patient engagement in early dialogues to ensure evidence generated in the clinical trials reflects outcomes of relevance to patients
- Generation and synthesis of robust PBE in a format useful for HTA
Finally, the creation of a multi-stakeholder group within the EU HTA to foster, strengthen, and evaluate patient involvement in EU HTA activities should be a critical path for the inclusion of patients in drug development and HTA in Europe.
The authors would like to acknowledge the following colleagues for their contributions to this article: Kevin Marsh, Executive Director Commercial Strategy & New Product Development, Patient-Centered Research, Evidera; Matthew Bending, PhD, Executive Director of HTA Strategy and UK Practice Lead, Market Access Consulting, Evidera; and Erem Latif, Director Patient Engagement, Patient-Centered Research, Evidera.
References
- National Health and Medical Research Council. NHMRC Levels of Evidence and Grades for Recommendations for Developers of Clinical Practice Guidelines. December 2009. Available at: http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=AEFFDA62A5245D6D07F060B56789ED5A?doi=10.1.1.177.4984&rep=rep1&type=pdf. Accessed September 5, 2019.
- Health Technology Assessment International. HTA Glossary. Available at: http://htaglossary.net/HomePage. Accessed September 5, 2019.
- Hutton J, McGrath C, Frybourg JM, Tremblay M, Bramley-Harker E, Henshall C. Framework for Describing and Classifying Decision-Making Systems using Technology Assessment to Determine the Reimbursement of Health Technologies (Fourth Hurdle Systems). Int J Technol Assess Health Care. 2006 Winter;22(1):10-8.
- Boudes M, Robinson P, Bertelsen N, et al. What Do Stakeholders Expect from Patient Engagement: Are These Expectations being Met? Health Expect. 2018 Dec;21(6):1035-1045.
- Weeks L, Polisena J Scott AM, Holtorf AP, Staniszewska S, Facey K. Evaluation of Patient and Public Involvement Initiatives in Health Technology Assessment: A Survey of International Agencies. Int J Technol Assess Health Care. 2017 Jan;33(6):715-723. doi: 10.1017/S0266462317000976. Epub 2017 Nov 10.
- Abelson J. Patient Engagement in Health Technology Assessment: What Constitutes ‘Meaningful’ and How We Might Get There. J Health Serv Res Policy. 2018 Apr;23(2):69-71. doi: 10.1177/1355819618756936. Epub 2018 Feb 7.
- Patients Active in Research and Dialogues for an Improved Generation of Medicine (PARADIGM). Available at: https://imi-paradigm.eu/. Accessed September 9, 2019.
- European Network for Health Technology Assessment (EUnetHTA). Available at: https://www.eunethta.eu/about-eunethta/history-of-eunethta/. Accessed September 9, 2019.
- Community and Patient Preference Research (CaPPRe). Patient Voice Initiative. Available at: https://www.cappre.com.au/PatientVoiceInitiative. Accessed September 9, 2019.
- Hunter A, Facey K, Thomas V, et al. EUPATI Guidance for Patient Involvement in Medicines Research and Development: Health Technology Assessment. Front Med (Lausanne). 2018 Sep 6;5:231. doi: 10.3389/fmed.2018.00231. eCollection 2018.
- Perfetto EM, Burke L, Oehrlein EM, Epstein RS. Patient-Focused Drug Development: A New Direction for Collaboration. Med Care. 2015 Jan;53(1):9-17. doi: 10.1097/MLR.0000000000000273.
- US Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. December 2009. Available at: https://www.fda.gov/media/77832/download. Accessed September 9, 2019.
- US Food and Drug Administration. Patient-Focused Drug Development: Collecting Comprehensive and Representative Input – Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Available at: https://www.fda.gov/media/139088/download. Accessed September 9, 2019.
- Sarri G, Kenny J, Freitag, et al. PHP315 – How Frequently is Patient Experience Formally Assessed in Health Technology Assessments? Results from A Systematic Literature Review. Value Health. October 2018;21(3):S204. doi.org/10.1016/j.jval.2018.09.1209.
- Single ANV, Facey KM, Livingstone H, Silva AS. Stories of Patient Involvement Impact in Health Technology Assessments: A Discussion Paper. Int J Technol Assess Health Care. 2019;35(4):266-272. doi: 10.1017/S0266462319000552. Epub 2019 Jul 24.
- Swedish Council on Health Technology Assessment. Self-harm: Patients’ Experiences and Perceptions of Professional Care and Support [Internet]. Stockholm: Swedish Council on Health Technology Assessment (SBU); 2015 Sep. SBU Alert Report No. 2015-04.
- NHS Scotland. Patient Information Leaflet. Understanding Your Chronic Wound Dressings, Management and Wound Infection. Available at: https://ewma.org/fileadmin/user_upload/EWMA.org/EWMA_Patient_ressources/Understanding-your-chronic-wound.pdf. Accessed September 9, 2019.
- CADTH. Interventions for Obstructive Sleep Apnea. February 23, 2016. Available at: https://www.cadth.ca/interventions-obstructive-sleep-apnea. Accessed September 9, 2019.
- National Institute for Health and Care Excellence (NICE). TNF-alpha Inhibitors for Ankylosing Spondylitis and Non-Radiographic Axial Spondyloarthritis. 2016 February 1. Available at: https://www.guidelinecentral.com/summaries/tnf-alpha-inhibitors-for-ankylosing-spondylitis-and-non-radiographic-axial-spondyloarthritis#section-date. Accessed September 9, 2019.
- EMA. Involvement of Patient Representatives in Scientific Advice Procedures at EMA. Available at: https://www.ema.europa.eu/en/documents/other/involvement-patient-representatives-scientific-advice-procedures-european-medicines-agency_en.pdf. Accessed September 9, 2019.
- EUPATI. Guidance for Patient Involvement in HTA. Available at: file:///W:\Patient%20Involvement%20in%20HTA\References\EUPATI%20guidance%20for%20patient%20involvement%20in%20HTA.pdf. Accessed September 9, 2019.
- Health Technology Assessment international (HTAi). Values and Standards for Patient Involvement in HTA. Available at: https://htai.org/interest-groups/pcig/values-and-standards/. Accessed September 9, 2019.
- Patient Focused Medicines Development. Patient Engagement Industry Training. Available at: https://patientfocusedmedicine.org/patient-engagement-industry-training/. Accessed September 13, 2019.
- EURORDIS Community Advisory Board (CAB) Programme. Available at: https://www.eurordis.org/content/eurordis-community-advisory-board-cab-programme. Accessed September 9, 2019.
- COMET Initiative. Available at: http://www.comet-initiative.org/. Accessed September 9, 2019.
- Health Technology Assessment international (HTAi). For Patient Groups and Individual Patients. Available at: https://htai.org/interest-groups/pcig/resources/for-patients-and-patient-groups/. Accessed September 13, 2019.
- EUnetHTA. HTA Core Model®. Available at: https://www.eunethta.eu/hta-core-model/. Accessed September 9, 2019.
- PREFER Patient Preferences. Available at: https://www.imi-prefer.eu/. Accessed August 30, 2019.
- European Medicines Agency. Human Regulatory: Clinical Trial Regulation. Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials/clinical-trial-regulation. Accessed September 9, 2019.
For more information, please contact us.