SPRING 2019, THE EVIDENCE FORUM, WHITE PAPER
 Andrew Bevan, MSc, CBiol, MRSB Director, Project Management Peri- and Post-Approval Operations Evidera |  Moira Ringo, PhD, MBA Senior Consultant Real-World Evidence Evidera |  Leona Fitzgerald, PhD Senior Director Regulatory Affairs PPD |  Fiona Kearney, MSc Senior Director, Project Management Peri- and Post-Approval Operations Evidera |  Delphine Saragoussi, MD, MScPH Research Scientist Real-World Evidence Evidera |
Introduction
The increasing development of orphan drugs and precision medicine has led to novel needs in terms of real-world evidence generation. A key area recently highlighted in the FDA’s updated draft guidance on rare diseases1 is the recommendation of natural history studies to better characterize patient populations and delineate target populations. Natural history studies are epidemiological studies that focus on describing the frequency, features, and evolution of a disease by collecting real-world data from groups of patients suffering from this disease. These studies are often performed by biotech and pharmaceutical companies early in the clinical development process to support and guide the design of clinical trial and drug development studies.
In the last few years, natural history studies have started to include genetic testing to describe specific genetic profiles as part of the features of the patient population, or as a screening criterion to identify the target population. The introduction of genetic testing within a fully non-interventional setting poses regulatory and ethical issues that are addressed differently by approval bodies (ethics committees, regulatory agencies, privacy committees, etc.) across the globe, highlighting the need for ongoing interpretation of the current regulations.
Natural History Studies and Genetic Biomarkers
Increased focus on rare diseases and precision medicine
The recent wave of new product introductions in rare diseases and precision medicine (including targeted oncology indications) has allowed improved outcomes for patients who would otherwise face grim prognoses. However, health system budgets have not necessarily adjusted to the high prices and to the increasing number of available therapies in these categories. This has led to a need for “triage” strategies that allow payers to select therapies that do the most good for the least utilization and cost.
There are many rare disease and advanced oncology therapies currently available, most of which are extremely costly. In 2018, the US approved 34 novel therapies for rare diseases, comprising 58% of new drug approvals last year2; this contrasts with 9 rare disease approvals in 2013 (See Figure 1).3 According to EvaluatePharma,4 rare disease and targeted oncology therapy sales are predicted to have 11-12% compound adjusted growth rates through 2024, which is more than double that for other prescription drugs. This growth is expected to continue as high financial returns will fuel more development and investment, which is expected to fuel more drug approvals and launches.
These factors combine to create a financial risk to payers. Consequently, payers manage the financial impact by restricting eligibility and reimbursement only to patients who are likely to have a significant benefit over standard of care.
Figure 1. US FDA Approvals of Novel Therapies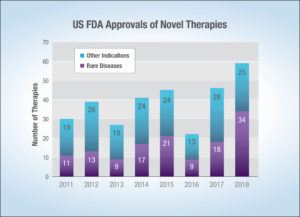
The role of natural history studies in the drug development strategy
The increased attention from biopharmaceutical companies and payers on rare disease and orphan drugs means there is a greater need to be able to accurately define the profile, characteristics, and disease outcomes of the target patient populations. This is where natural history studies (See Panel 1) play a key role for both stakeholders.
On one hand, natural history studies can inform clinical product development by:
- Providing better insights into disease characteristics, patient populations, and identification of disease subtypes
- Identifying the most sensitive and relevant endpoints or the optimal duration of follow-up
- Identifying patients eligible for clinical trials
- Serving as an historical comparator in case of single arm trials
Panel 1. Natural History Studies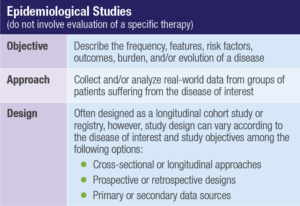
On the other hand, natural history studies help payers “triage” care to patients most likely to benefit from therapies by:
- Assessing disease burden in real-world clinical practice under standard of care
- Identifying and describing sub-types of a disease that have a higher burden
- Identifying patient sub-populations who are less or more likely to respond to current therapies (See Figure 2)
- Identifying patient sub-populations that are likely to have the greatest benefit versus risk with new therapies
Figure 2. Case Study: Characterizing Potential Responders to Enhance Differentiation and Optimize Market Access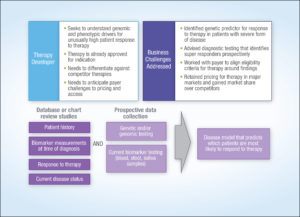
The emergence of genetic biomarkers in real-world evidence generation
Biomarkers are an integral part of natural history studies for rare disease and advanced oncology therapies. This is driven by the nature of the diseases since many rare diseases are caused by inherited genetic mutations. The number and specific type of mutations can be highly predictive of disease severity and response to treatment. There is also a trend towards identifying biomarker-based subgroups of patients suffering from a more common disease that could present with characteristics such as a worse prognosis or being difficult to treat and that could be identified as target populations for targeted treatments.
The FDA now considers biomarker identification or validation as a full part of natural history studies in rare diseases.1 This introduces a new paradigm into the regulatory and operational aspects of running natural history studies.
Regulatory Interpretation of Genetic Testing in Natural History Studies
Non-interventional vs. interventional
Real-world evidence generation is clearly differentiated from clinical trials, with its main feature being that patients are treated and monitored according to routine clinical practice and not according to any study protocol-defined procedures. However, in the perspective of maximizing the protection of patients, regulators and ethics committees have been issuing guidance around operationalization of real-world evidence generation. Regulations and guidance vary across geographies and are continuously evolving as new information becomes available and healthcare systems mature.
In practice, the main (but not unique) feature determining the classification of a real-world study as interventional or non-interventional is its objective and whether it focuses on observing the characteristics of a disease or on observing the effects of a drug.5,6 Natural history studies are typically focused on observing the characteristics of a disease. However, the need for specific tests such as questionnaires or non-routine biological samples is potentially another criterion for classification and happens to be interpreted differently across geographies. Typically, genetic testing can be done either via buccal swab (considered non-invasive) or more often via blood sample (considered invasive). According to the disease being studied and the regional/local regulations, blood sampling may be considered either a routine diagnosis or monitoring procedure, or as a protocol-driven, non-routine procedure.
When setting up a natural history study, it is helpful to understand the probable classification of the study according to geography as well as the corresponding regulatory pathway for planning and organization purposes.
Genetic testing
There currently is no consensus definition of genetic testing, despite many organizations and governments who have voiced a desire for such an agreed upon definition. Furthermore, notable bodies such as the International Conference on Harmonization (ICH) and the US Food and Drug Administration (FDA) have not published any definitive guidance on genetic testing in the context of clinical research, therefore, it is not surprising that the implementation of regulations for genetic testing is diverse and confined to individual country statutes. There are some areas where consensus is emerging,7 but clearly there is still much to be accomplished towards the harmonization of guidance and regulations.
Figure 3 visually summarizes the relevance and connection of the elements that drive the regulatory and ethics pathways, showing how study objectives, geography, and genetic testing all play a role in the type of studies required for treatments of rare diseases.
Figure 3. Drivers of the Regulatory and Ethics Pathways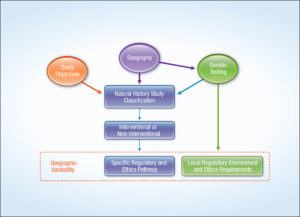
Other considerations
In addition, if the natural history study is to serve as historical control to a one-arm clinical trial, it is recommended to seek preliminary regulatory agency agreement to such designs ahead of submitting final protocol.
Operational Approach to Natural History Studies with Genetic Testing
Case study
Figure 4 presents the case of a multi-country natural history study including genetic testing and how the different countries classified each study, together with the consequences in terms of regulatory process and ethics submission. This example shows the variability in study classification across geographies and within member states of the European Union (EU). Significant differences can be seen in certain geographies, particularly in France where we see non-routine biological sample collection for prospective research defining the study as interventional.
Figure 4. Case Study: Natural History Study in a Rare Disease Indication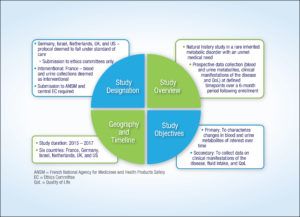
Practical implications
In practical terms, the lack of harmonized regulations regarding biomarker and genetic sampling, particularly in the context of natural history studies, means that product developers and researchers need to tread cautiously when planning research and carefully assess the regulatory landscape on a case-by-case, country-by-country basis to determine how to proceed. For example, regulatory requirements in France for studies involving biomarker genetic sample collection are clear, and both Ethics Committee (EC) and Regulatory Authority (RA) approval are required, whereas in other countries where regulatory requirements for genetic testing are less defined and therefore open to interpretation, advice may need to be sought from regulatory bodies beforehand in order to ensure the correct pathway is taken to secure approval.
Despite the current lack of guidance, genetic testing implies some responsibilities for the study sponsor and potential consequences for the patient that need to be considered. The ethical and patient care implications of genetic testing might expand beyond the scope of the proposed research. For example, if the patient is determined to have a confirmed or suspected pathogenic mutation:
- Should the patient be informed? Who should inform, counsel, and manage the patient?
- What happens if the significance of the finding is unclear at that time, or the significance only becomes clear many years after the patient was tested?
- Is there an obligation to provide additional patient monitoring or management because of a genetic testing result? Who decides what is appropriate for each patient?
- Is there an obligation to inform and/or test family members for the mutation (e.g., cascade screening)?
Conclusion
The emergence of new therapies focusing on rare diseases and targeted oncology indications leads to new needs in terms of real-world evidence generation, with the development of a new kind of natural history study that includes biomarker testing. These new needs challenge the regulatory framework that was initially shaped by clinical trials and traditional non-interventional studies, and this new situation translates into a diverse and moving regulatory environment for this new type of study. A timely and integrated multidisciplinary approach based on consistent strategic (why), scientific (what), and operational (how) considerations allows for anticipation of challenges and planning of preparatory steps for successful implementation of such studies.
References
- US Food and Drug Administration. Rare Diseases: Common Issues in Drug Development Guidance for Industry – Draft Guidance. February 2019. Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM629579.pdf. Accessed February 7, 2019.
- US Food and Drug Administration, Center for Drug Evaluation and Research. Advancing Health Through Innovation: 2018 New Drug Therapy Approvals. January 2019. Available at: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/UCM629290.pdf. Accessed February 22, 2019.
- US Food and Drug Administration. Novel Drug Approvals for 2018. Available at: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm592464.htm. Accessed February 22, 2019.
- EvaluatePharma. Orphan Drug Report 2018, 5th Edition, May 2018. Available at: http://info.evaluategroup.com/rs/607-YGS-364/images/OD18.pdf. Accessed February 7, 2019.
- Official Journal of the European Union. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human use, and Repealing Directive 2001/20/EC. Available at: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_2014_536/reg_2014_536_en.pdf. Accessed February 7, 2019.
- ClinicalTrials.gov. Glossary of Common Site Terms. Available at: https://clinicaltrials.gov/ct2/about-studies/glossary. Accessed February 7, 2019.
- Council of Europe. The Oviedo Convention: Protection Human Rights in the Biomedical Field. Available at: https://www.coe.int/en/web/bioethics/oviedo-convention. Accessed February 22, 2019.
For more information, please contact
[email protected], [email protected],
[email protected], [email protected], or [email protected]




