SPRING 2021, THE EVIDENCE FORUM, WHITE PAPER
 Alex Ward, PhD, MRPharmS Executive Director, Senior Research Leader Evidence Synthesis, Modeling & Communication Evidera, a PPD business |  Conor Chandler, BS Senior Research Associate Evidence Synthesis, Modeling & Communication Evidera, a PPD business |  Henri Folse, PhD Research Scientist Evidence Synthesis, Modeling & Communication Evidera, a PPD business |  Péter Gál, MSc Research Scientist, Research Director Evidence Synthesis, Modeling & Communication Evidera, a PPD business |  Ameya Chavan, MS Research Associate Evidence Synthesis, Modeling & Communication Evidera, a PPD business |
Introduction
Parkinson’s disease (PD) is a progressive disease that leads to both motor and non-motor symptoms. There is currently no disease-modifying therapy (DMT) available for the treatment of patients with PD, but new therapies are being studied and entering clinical trials.1 Most of these clinical trials will use the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) subscales as primary outcomes, and plan to study patients recently diagnosed with PD.
Many health economic models have assessed the cost-effectiveness of existing treatments for PD. According to the findings of a systematic literature review, most published models used a Markov cohort approach, where the Hoehn and Yahr scale was commonly used to define and model transitions between health states. As the models identified did not consider MDS-UPDRS, a need was identified for a de novo model to support the assessment of the health economics of DMTs administered soon after diagnosis. The de novo model was built upon the MDS-UPDRS scales to align with the clinical trial designs.
This article describes a new model framework developed to simulate PD progression from diagnosis, capturing both motor and non-motor symptoms, the impact on health outcomes, and the associated costs. This simulation framework can be used to predict the long-term clinical outcomes of new treatments, such as DMTs, in addition to the current standard of care, and can be leveraged to conduct cost-effectiveness analyses and clinical trial simulations.2,3
In this article we first outline the model’s structure, data sources, and validation, and then discuss the potential applications for this disease simulator to inform internal decision-making, trial design, and strategic planning early in the development of DMTs.
Model Framework
The model was constructed as an individual patient simulation to simulate the clinical and economic outcomes of patients newly diagnosed with PD.
- Characterizes disease progression in terms of sequential changes in key clinical scales using a set of interrelated predictive equations for progression of MDS-UPDRS and UPDRS subscale scores
- Captures both the short-term benefits of symptomatic treatments, and their long-term limitations, such as increasing off-time and the associated complications of therapy
- Predicts the long-term benefits of DMTs due to slowing the rate of disease progression as distinct from symptomatic improvements
The simulated patient characteristics influencing disease progression include age, sex, and disease duration, as well as the initiation of dopaminergic medications or advanced therapies (e.g., deep brain stimulation). This simulation was implemented in Microsoft Excel® and uses the discretely integrated condition event (DICE) approach.4 The progression and management of PD is therefore conceptualized as a combination of evolving conditions (age, MDS-UPDRS, UPDRS, Hoehn and Yahr [HY], costs, and utilities) and events (distinct points in time where conditions change, such as medications, discontinuation, institutionalization, or death).
Disease Progression
PD is a slow progressing disease and therefore no single dataset with longitudinal MDS-UPDRS or UPDRS data was available that followed patients from diagnosis to the advanced stages of the disease. New predictive equations were developed by analyzing two data sources to model disease progression for newly diagnosed patients. These were then combined with additional published sources to inform long-term progression, mortality, utilities, and costs.5-7
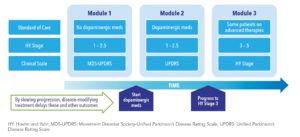
- The model has three modules for distinct phases of the disease progression, each based on a different data source (See Figure 1). Mappings between various scales maintain internal consistency.
- A series of new predictive equations were developed based on longitudinal data obtained from Parkinson’s Progression Markers Initiative (PPMI)8 for MDS-UPDRS scales and the National Institute of Neurological Disorders and Stroke (NINDS) Exploratory Trials in PD Long-Term Study1 (NET-PD LS-1) for the UPDRS scales.9,10
- Profiles of newly diagnosed patients were generated by jointly sampling correlated characteristics from PPMI (mean age 61.2 years, male 65%, duration six months, treatment-naïve).
- A new predictive equation was developed to estimate EQ-5D-3L-derived utilities, capturing motor and non-motor symptoms.
- Progression from HY Stage 3 was based on study published by Johnson et al.11
Validation
Extensive validation of the projections and technical verification was a key step in the development of this new disease simulator. The analyses illustrated the model appropriately simulated progression for both treatment-naïve and treatment-experienced patients.
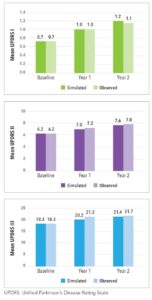
The progression equations were first individually confirmed by comparing the predicted and observed scores each year post-baseline to ensure the predictions were aligned. These equations were then implemented in the model, and the simulated outcomes were confirmed to align with the observed longitudinal data for three cohorts:
- Treatment-naïve patients (PPMI data)
- Patients on PD medication at baseline (NET-PD LS-1 data)
- Treatment-naïve patients based on an external source (PRECEPT data)12
In the simulation, the predicted values from one equation are used as predictors for correlated measures; therefore, this step assessed the joint validity of the equations once implemented in the model. One limitation to the project was that the PPMI data set was used to develop the functions for projecting treatment-naïve progression, as well as for validation of the model. However, the equations developed from the NET-PD LS-1 for progression after initiation of dopaminergic medications were validated against an external data source (See Figure 2).
Discussion
The newly developed equations supported a de novo model framework suitable for conducting simulations from early in the disease and captures the progression of both motor and non-motor symptoms. Additional validations and refinements to this simulator are ongoing. The model can be used early in a drug development program to conduct scenario analyses to inform internal decision-making and strategic planning. This might include simulating the potential benefits of a new DMT and how certain design decisions could impact the likelihood of success of a trial. The influence of varying key clinical trial design assumptions can be simulated such as:
- Inclusion or exclusion criteria applied to select specific sub-populations (e.g., treatment-naïve, age range, HY stages, etc.)
- Mean change in MDS-UPDRS or UPDRS (individual subscales or combinations)
- Trial duration
- Sample size and dropout rates
This tool facilitates conducting exploratory analyses by varying key parameters, such as the durability of health benefits (i.e., immediate loss or gradual waning of benefits) and treatment stopping rules. The results from these scenarios can help to understand the key drivers of cost-effectiveness and identify important data gaps to inform evidence generation planning for each market. Assessing the likely pricing to be cost-effective at various willingness-to-pay thresholds can often be informative. For example, running scenarios can generate an evaluation of the economically justifiable price (i.e., the price at which the estimated incremental cost-effectiveness ratio is equal to the selected cost-effectiveness thresholds).
The model was systematically validated against the source data and an external data set. Long-term access to additional data sources from other populations would provide a more complete understanding of the generalizability of the equations developed. This project has allowed us to construct a new framework that is extremely flexible and customizable, allowing users to generate their own scenarios without the need to interact with the complex programming within the model.
This framework facilitates running simulations of a proposed clinical trial protocol, and comparison of the likely results, with many alternative options and assumptions for the design, patient population, and outcome measures. Early modeling can also support identifying gaps in the data available and defining the critical questions to prioritize addressing in order to meet the requirements of health technology assessment groups and help plan for payer discussions. These types of simulations early in development can support optimizing value demonstration for new innovative therapies for a complex disease and increase the likelihood the final value proposition will be accepted by both regulators and payers.
References
- Dawson VL, Dawson TM. Promising Disease-Modifying Therapies for Parkinson’s Disease. Sci Transl Med. 2019 Nov 27;11(520):eaba1659. doi: 10.1126/scitranslmed.aba1659.
- Gal P, Folse H, Chavan A, Chandler C, Ward A. Clinical Trial Simulation to Support the Design of a Randomized Controlled Trial of a Hypothetical Disease-Modifying Treatment for Parkinson’s Disease. 2nd Advances in Alzheimer’s and Parkinson’s Therapies an AT-AD/PDTM Focus Meeting, 2-5 April 2020, Vienna, Austria.
- Chandler C, Gal P, Folse H, Chavan A, Ward A. Cost-Effectiveness Analysis of a Hypothetical Disease Modifying Therapy for Parkinson’s Disease. International Society for Pharmacoeconomics and Outcomes Research Annual International Meeting, 14-18 November, 2020, Milan, Italy. Value in Health 2020 December; 23 Suppl 2 (S633).
- Caro JJ. Discretely Integrated Condition Event (DICE) Simulation for Pharmacoeconomics. Pharmacoeconomics. 2016 Jul;34(7):665-72. doi: 10.1007/s40273-016-0394-z.
- Kaltenboeck A, Johnson SJ, Davis MR et al. Direct Costs and Survival of Medicare Beneficiaries with Early and Advanced Parkinson’s Disease. Parkinsonism Relat Disord. 2012 May;18(4):321-6. doi: 10.1016/j.parkreldis.2011.11.015. Epub 2011 Dec 16.
- Lowin J, Sail K, Baj R et al. The Cost-Effectiveness of Levodopa/Carbidopa Intestinal Gel Compared to Standard Care in Advanced Parkinson’s Disease. J Med Econ. 2017 Nov;20(11):1207-1215. doi: 10.1080/13696998.2017.1379411. Epub 2017 Sep 21.
- Findley LJ, Wood E, Lowin J, Roeder C, Bergman A, Schifflers M. The Economic Burden of Advanced Parkinson’s Disease: An Analysis of a UK Patient Dataset. J Med Econ. 2011;14(1):130-9. doi: 10.3111/13696998.2010.551164.
- Parkinson’s Progression Markers Initiative. Available at: http://www.ppmi-info.org/. Accessed March 1, 2021.
- Chandler C, Franco-Villalobos C, Wang Y, Gal P, Folse HJ, Ward A. Development of a Statistical Model to Predict EuroQol Five Dimensions (EQ-5D) Utilities in Parkinson’s Disease. Value in Health ISPOR. 2020 May 1;23 (Suppl 1):S9-S10. doi: 10.1016/j.jval.2020.04.052.
- Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators, Kieburtz K, Tilley BC et al. Effect of Creatine Monohydrate on Clinical Progression in Patients with Parkinson Disease: A Randomized Clinical Trial. JAMA. 2015 Feb 10;313(6):584-93. doi: 10.1001/jama.2015.120.
- Johnson SJ, Diener MD, Kaltenboeck A, Birnbaum HG, Siderowf AD. An Economic Model of Parkinson’s Disease: Implications for Slowing Progression in the United States. Mov Disord. 2013 Mar;28(3):319-26. doi: 10.1002/mds.25328. Epub 2013 Feb 12.
- Parkinson Study Group PRECEPT Investigators. Mixed Lineage Kinase Inhibitor CEP-1347 Fails to Delay Disability in Early Parkinson Disease. Neurology. 2007 Oct 9;69(15):1480-90. doi: 10.1212/01.wnl.0000277648.63931.c0. Epub 2007 Sep 19.