SPRING 2020, THE EVIDENCE FORUM, WHITE PAPER
 |
| Mariah Baltezegar, MBA Executive Director and Head Peri- and Post-Approval Virtual Trials Real-World Evidence Evidera |
The COVID-19 pandemic has washed over the world in waves and affected every aspect of life, including most obviously, healthcare. The impact is not only seen in relation to coronavirus patients and studies, but the ripple effect is also seen across all clinical and real-world studies. Several key factors have disrupted research efforts, including shelter-at-home mandates, limited access to healthcare facilities, patients’ comfort level in participating in studies, and the shift in priorities and capacity of healthcare providers. These types of disruptions lead to some key challenges. For example, patients cannot visit sites to have their standard of care or protocol-defined safety assessments performed; patients’ ability to visit sites for clinical or patient-reported outcome assessments are hindered; and there is a decrease in patient recruitment and retention rates. Patient safety is always the main priority of our industry, and we also need to continue to collect study dictated data as much as possible. To do this, we need to figure out ways to ensure patient safety in the current global scenario and mitigate the impact on study disruption.
How Regulators are Advising Stakeholders in These Dynamic Times
Globally, regulatory and data privacy guidance is evolving to address the current challenges faced by the industry. While specific guidance and actions may differ among regulatory agencies and ethics committees, it’s clear that patient safety comes first. Guidance from the European Medicines Agency (EMA),1 US Food and Drug Administration (FDA),2 and Advarra,3 a US Central Institutional Review Board (IRB), all mention the use of telemedicine and virtual visits to continue communication with patients and maintain engagement to ensure patient safety. The Italian Medicines Agency has allowed for a special provision for sponsors to directly engage specialized agencies such as home nursing to support management of patients that previously had to be contracted separately and directly with the Principal Investigator (PI).4 Regulators continue to apply pragmatism to ensure continued care and patient safety.
Strategies to Bring a Study to the Patient
The continuum of data collection includes everything from the traditional brick and mortar approach, where a patient must go to a research site for all study assessments (posing the highest burden to patients), to a fully virtual, decentralized or metasite approach, where a patient never has to go to a research site for assessments (posing the lowest burden). Given the constraints COVID-19 is placing on the industry right now, the high burden brick and mortar approach is unachievable. Fortunately, virtual enablement approaches are available and solve many of the challenges the industry is currently experiencing.
While physical in-person visits to a study site may not be possible, there are alternate solutions possible to address each need, with some strategies being immediate and relatively short-term, while others are longer-term solutions.
- Televisits are available for patients who are unable to visit their healthcare providers to have standard of care, protocol-defined safety assessments performed. These visits enable healthcare providers to physically see a patient and perform assessments via video conference for both standard of care and protocol-defined assessments. This is being applied both to research activities as well as routine healthcare.
- Remote e-signature consent can be employed to acquire remote consent signatures for patients who do not have the appropriate consent in place or cannot visit sites to be consented. It is important to note that issues related to consent are continually evolving. For example, Advarra released additional guidance on 27 March 2020 noting that re-consent is not necessary unless the research challenge changes such that the original consent is no longer valid (e.g., re-consent is not necessary when changing from clinic visits to remote visits).
- Direct to patient approaches, such as electronic clinical outcomes assessments (eCOAs), electronic patient-reported outcomes (ePROs), devices and wearables, and home nurses and phlebotomists are options for patients who cannot visit their study site for clinical or patient-reported outcome assessments.
- Direct to patient supplies provide study medication or other supplies necessary to conduct study assessments via direct shipments to the patient’s home when patients are unable to visit a site to replenish their clinical supplies.
It is possible to leverage individual solutions or bring these solutions together in a metasite model with a digitally enabled platform. With the metasite model, the burden is removed from the site staff who may be involved in frontline care during this pandemic and bring the study directly to the patient, allowing them to participate from the comfort of their homes. Table 1 summarizes these digital and other solution options.
Table 1. Solutions to Address Challenges Faced by Patients Unable to Visit Study Sites and Reduce Site Burden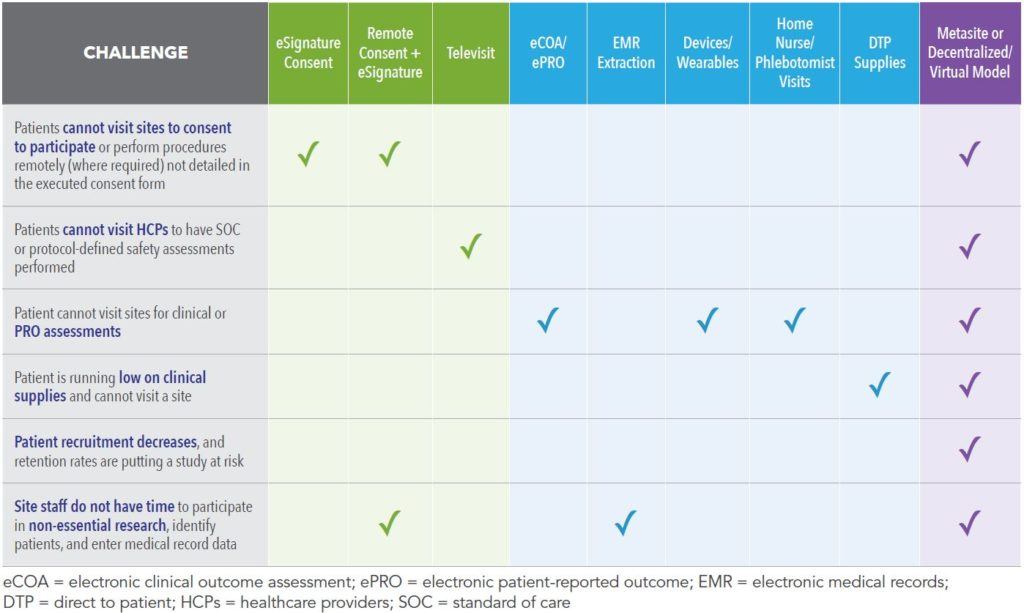
Additional Strategies for RWE Data Collection
In addition to the solutions already presented, there are other strategies that minimize burden on patients, caregivers, and healthcare providers that may meet some of the needs brought on by COVID-19. Electronic medical record (EMR) extraction uses existing health information exchange (HIE) technology to connect clinical sites’ EMR data and pre-enable them for research. This streamlined approach to accessing sites’ rich EMR data answers specific research questions in a more rapid, automated, and repeatable manner and can eliminate the need for site staff to perform data transcription and free site staff to perform other activities while enabling continued data collection.
Alignment with integrated delivery networks (IDNs) allows recruitment of patients at the point of routine care versus traditional research centers. This strategy utilizes large healthcare delivery organizations that either own or manage multiple points of patient care (e.g., hospitals, physician practices, long-term care facilities), allowing for rapid feasibility as well as patient identification through centralized EMRs and enabling e-recruitment of potential patients or study participants. Existing registry data can also be leveraged to map study objectives, assessments, and measures across data sources. For example, the existing data sets being collected on COVID-19 in various geographies and formats and for various purposes can be used to answer research questions.
Real-World Examples of Study Engineering to Maintain Continuity of Data Collection
Case Study One
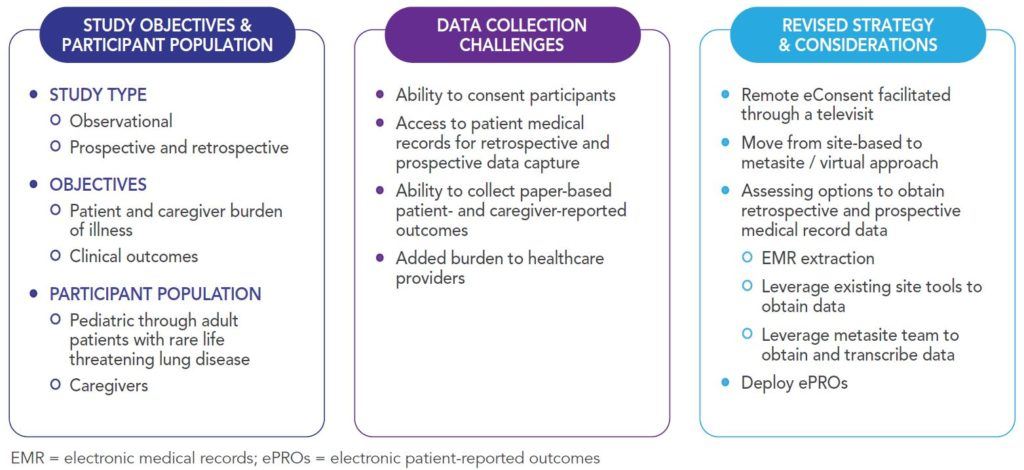
Concerns arose about the continuity of a current, ongoing observational study given the current pandemic and resulting shifts in priorities of healthcare providers, access to healthcare facilities, potential future local restrictions, and patient and caregiver preferences given the at-risk status of the patient population in the study. By evaluating the needs and using the options identified earlier in this article, a revised strategy was developed for this study. Figure 1 outlines the study objectives, challenges, and revised strategy to avoid study delays.
Case Study Two
In this example, a prospective Phase III study using an interventional oral therapy in dermatology needed to develop a rapid solution given a six-day lead time. This need was in a geographically sensitive area where patients had primary endpoint visits in the very short term. The primary endpoint is assessed via a scale that can only be performed visually, but the country is on complete lockdown, so face-to-face assessment was not possible. With time of the essence, this scenario required existing capabilities and partnerships, close relationships and alignment with all key stakeholders, and immediate mobilization for a successful outcome. Figure 2 identifies the study details, challenges, and solutions implemented.
While it is important to understand the available technology and how that technology can be deployed, it is equally, if not more important, to understand the relationships, processes, and limitations that support successful implementation.
Figure 2. Phase III Study Strategy to Ensure Continuity of Primary Endpoint Data Collection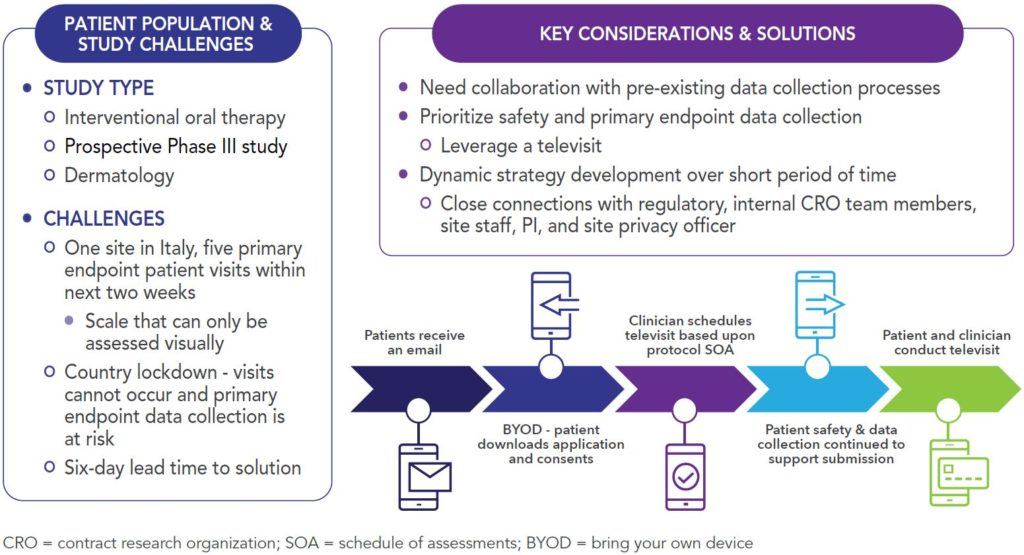
Conclusion
We need to be thoughtful and practical around collecting data in the current environment, while at the same time considering novel and alternative options to ensure clinical and real-world studies can continue. Regulators and other stakeholders are supportive of pragmatism while maintaining patient safety, both from a study data collection and virus exposure and risk minimization perspective. Decentralized solutions have been prioritized across the industry to address immediate needs for COVID-19 studies and other studies disturbed by the effects of the pandemic, but these solutions hold longer term potential. Outside of the current, urgent needs that digital solutions are helping to alleviate, these strategies offer the future standards for study development and allowing greater patient access and interactions that will expand the possibilities for future research.
References
- European Medicines Agency. Guidance on the Management of Clinical Trials During the COVID-19 (Coronavirus) Pandemic. Version 3. 28/04/2020. Available at: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf. Accessed April 28, 2020.
- US Food and Drug Administration. FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency: Guidance for Industry, Investigators, and Institutional Review Boards. March 2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency. Accessed April 28, 2020.
- Advarra. Remote Visits to Substitute for In-Office/Hospital/Clinic Visits. Available at: https://www.advarra.com/coronavirus-guidance/?utm_source=pr&utm_#remote-visits. Accessed April 28, 2020.
- The Italian Medicines Agency (AIFA). Management of Clinical Studies in Italy During an Emergency COVID-19 (Coronavirus Disease 19). Available (in Italian only) at: https://www.aifa.gov.it/documents/20142/871583/Comunicato_gestione_studi_clinici_in_emergenza_COVID-19_12.03.2020.pdf/9b3296a7-9935-f1c0-9489-0bfc87653bad. Accessed April 28, 2020.
For more information, please contact us
or [email protected]