JUNE 2019, POLICY TRENDS UPDATE
 |  |  |  |
| Amy Sood, PharmD Manager Scientific Advice CADTH | Michelle Mujoomdar, PhD Director Scientific Affairs CADTH | Matthew Bending, PhD Executive Director of HTA Strategy Market Access Consulting Evidera | Jennifer Boss, MSc Market Access Writer Market Access Communications and Policy Trends member covering Canada Evidera |
In April 2019, members of Evidera’s Policy Trends team had the opportunity to speak with scientific staff members from the Canadian Agency for Drugs and Technologies in Health (CADTH) to discuss new opportunities available through CADTH’s scientific advice services. Key points of interest from this discussion are outlined in this article.
What new opportunities for scientific advice now exist with CADTH?
CADTH recently launched two parallel scientific advice programs with the National Institute for Health and Care Excellence (NICE; February 2019)1,2 and Health Canada (regulatory agency; March 2019).3,4 Applications for both parallel scientific advice programs are now being accepted.
What are the processes for obtaining advice through these new parallel scientific advice programs?
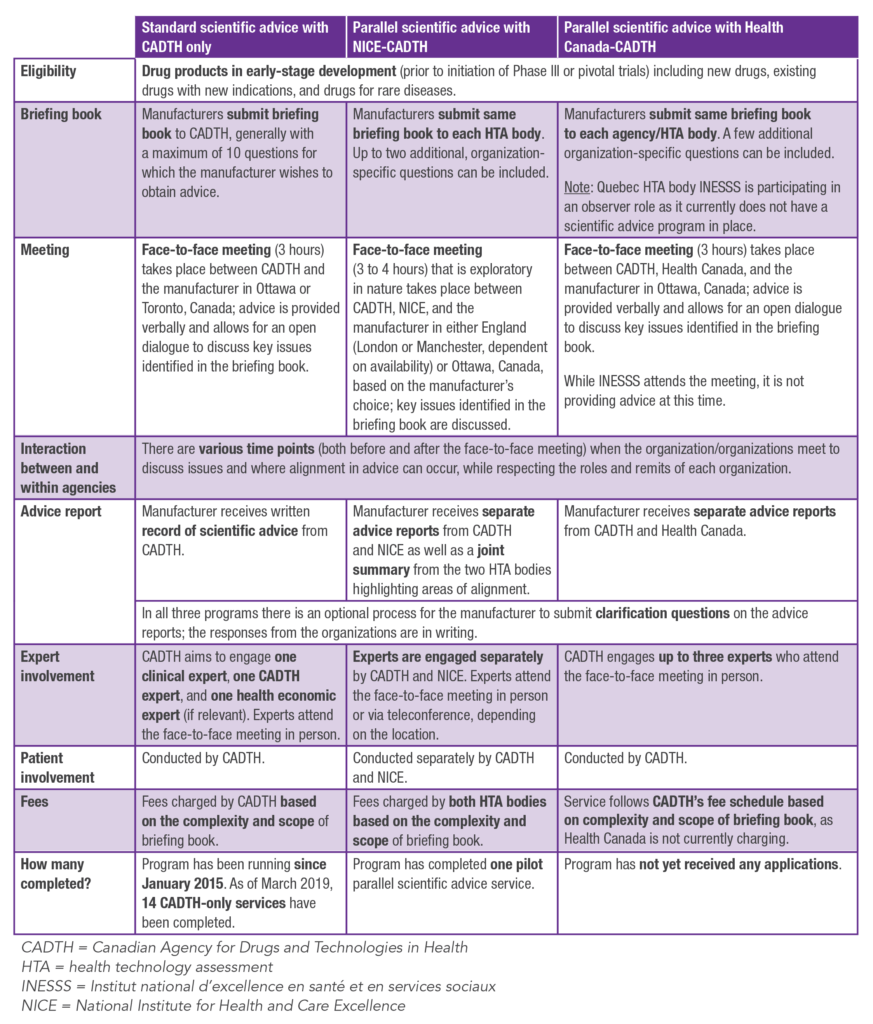
An overview of CADTH’s parallel scientific advice programs with NICE and Health Canada, as well as the standard scientific advice program with CADTH only5 is presented in Table 1. Both parallel scientific advice programs include submission of the same briefing book to each organization involved, a face-to-face meeting between the manufacturer and organizations, and provision of separate advice reports by each organization (plus a summary of alignment provided by the NICE-CADTH program only).
The parallel scientific advice processes allow manufacturers to confirm a single approach for their evidence generation plans that meets the needs of both organizations.
Slight differences between the parallel scientific advice programs include the fee schedule, format and location of the face-to-face meeting, timelines, and the summary of alignment which is provided by the CADTH-NICE program only.
Why is CADTH collaborating with NICE?
NICE was the first health technology assessment (HTA) body to establish an HTA scientific advice program in 2009 and has since become a well-established and experienced provider of scientific advice. CADTH’s standard scientific advice program launched in 2015 and was developed based on NICE’s program (in addition to consultation with other established scientific advice programs). Hence, NICE and CADTH’s programs have inherent similarities in how they provide advice. The parallel advice offering between CADTH and NICE allows for synergies between the two programs to streamline the process of obtaining advice in two distinct markets.
How does CADTH’s scientific advice program differ from others?
CADTH’s scientific advice differs from that of European HTA bodies in its provision of advice, as evident in CADTH’s standard scientific advice program. During the face-to-face meeting with CADTH, the manufacturer has the opportunity to verbally receive and discuss the actual draft advice with CADTH and raise any points of clarification in real time. This differs from other scientific advice programs where face-to-face meetings tend to occur at an earlier stage in the process and are more exploratory in nature, thus not providing manufacturers with the opportunity to discuss the drafted advice with the HTA body. Note that in all three of CADTH’s scientific advice programs, manufacturers have an opportunity to submit written clarification questions on the advice reports.
Other Canadian developments to watch for in the coming months
CANADA TO CREATE NEW AGENCY FOR DRUG EFFICACY EVALUATION AND PRICING NEGOTATION
As part of a plan to make high-cost prescription drugs more affordable for Canadians through the National Pharmacare initiative (for a public, universal prescription drug plan), the federal government announced the creation of the Canadian Drug Agency which will take a coordinated approach to evaluating drug efficacy and negotiating prices.6 While few details about the new agency have been released, it is suggested that the Canadian Drug Agency will build on the work of HTA agencies CADTH and INESSS, as well as the pan-Canadian Pharmaceutical Alliance which negotiates prices for prescription drugs on behalf of provincial and territorial drug plans.
Development of a national formulary listing has also been proposed as part of National Pharmacare, as well as a national strategy for high-cost drugs for rare diseases.
The final report by the Advisory Council on the Implementation of National Pharmacare is due to be released in spring 2019 (but has not been released at the time of this article).
References
- CADTH. New Opportunity: Parallel Scientific Advice from CADTH and NICE. February 6, 2019. Available at: https://cadth.ca/news/new-opportunity-parallel-scientific-advice-cadth-and-nice. Accessed May 16, 2019
- NICE. NICE Collaborates with the Canadian Agency for Drugs and Technology in Health to Offer Parallel Scientific Advice. February 6, 2019. Available at: https://www.nice.org.uk/news/article/nice-collaborates-with-the-canadian-agency-for-drugs-and-technology-in-health-to-offer-parallel-scientific-advice. Accessed May 16, 2019.
- CADTH. Health Canada and CADTH Launch New Initiative to Provide Early Parallel Scientific Advice. March 1, 2019. Available at: https://www.cadth.ca/scientific-advice. Accessed May 16, 2019.
- Health Canada. Notice to Industry: Health Canada and CADTH Launch New Initiative to Provide Early Parallel Scientific Advice. February 28, 2019. Available at: https://www.canada.ca/en/health-canada/corporate/transparency/regulatory-transparency-and-openness/improving-review-drugs-devices/notice-early-panel-scientific-advice.html. Accessed May 16, 2019.
- CADTH. Scientific Advice Program. May 12, 2019. Available at: https://www.cadth.ca/scientific-advice/about. Accessed May 16, 2019.
- Government of Canada. Investing in the Middle Class: Budget 2019. March 19, 2019. Available at: https://budget.gc.ca/2019/docs/plan/budget-2019-en.pdf. Accessed May 16, 2019.