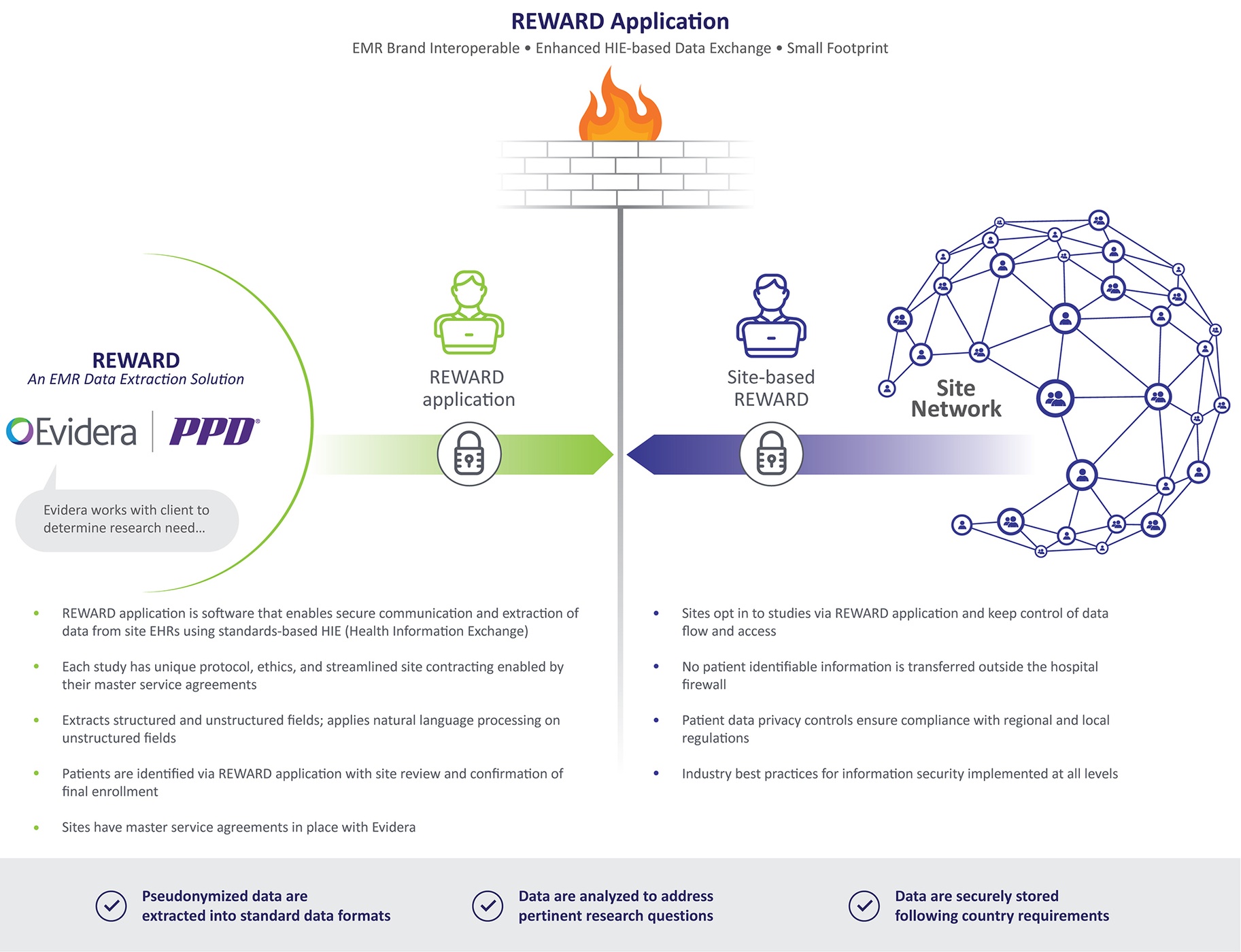
RWE Generation via Traditional and Secondary Data Sources May Not Always Be Enough
The need for RWE generation to satisfy a breadth of research questions as well as multiple stakeholder needs continues to grow. While the use of existing databases offers rapid insights, they often lack the clinical precision required to address increasingly sophisticated research questions. Traditional site-based studies offer richer clinical data, but they require manual processes that lengthen timelines and are prone to data entry errors. Technology-driven data collection offers many advantages to traditional methods for capturing data in observational studies. It facilitates extraction directly from the source to minimize data entry errors, thereby reducing the volume of data queries. It also streamlines data collection and cleaning processes, as data are extracted directly into standard formats conducible to analyses and its automation allows for efficient repeat data extractions.