FALL 2020, THE EVIDENCE FORUM, WHITE PAPER
 Jürgen Hummel, MSc Senior Director, Statistical Science PPD |  Rachael Song, MBA Associate Director, Hematology/Oncology Global Project Management PPD |  Dirk Reitsma, MD Vice President and Therapeutic Area Head, Oncology Global Product Development Evidera, a PPD business |  Kent Buhler, MBA Senior Director, Operations Strategy Lead, Hematology/Oncology PPD |  Song Wang, PhD Statistical Science Director PPD |
Oncology trials make up over a third of today’s pharmaceutical research pipeline, but conventional oncology drug development programs are often inefficient, expensive, and suffer from high failure rates.1 Of the oncology agents that enter Phase I trials, only about 3% eventually receive approval from the United States Food and Drug Administration (FDA).2
Just as other industries have moved toward more flexible methodologies that foster continual improvement and operational efficiencies, clinical development is slowly ramping up adoption of innovative designs after being encouraged by regulatory agencies to speed progress, reduce inefficiencies, and improve success rates.
This article focuses on the early stage of oncology trials where important decisions about dose selection and target indications that may have far-reaching consequences are made. We explore potential scientific and operational implications for two different well-established designs:
- The continual reassessment method (CRM), an adaptive design that identifies the target dose
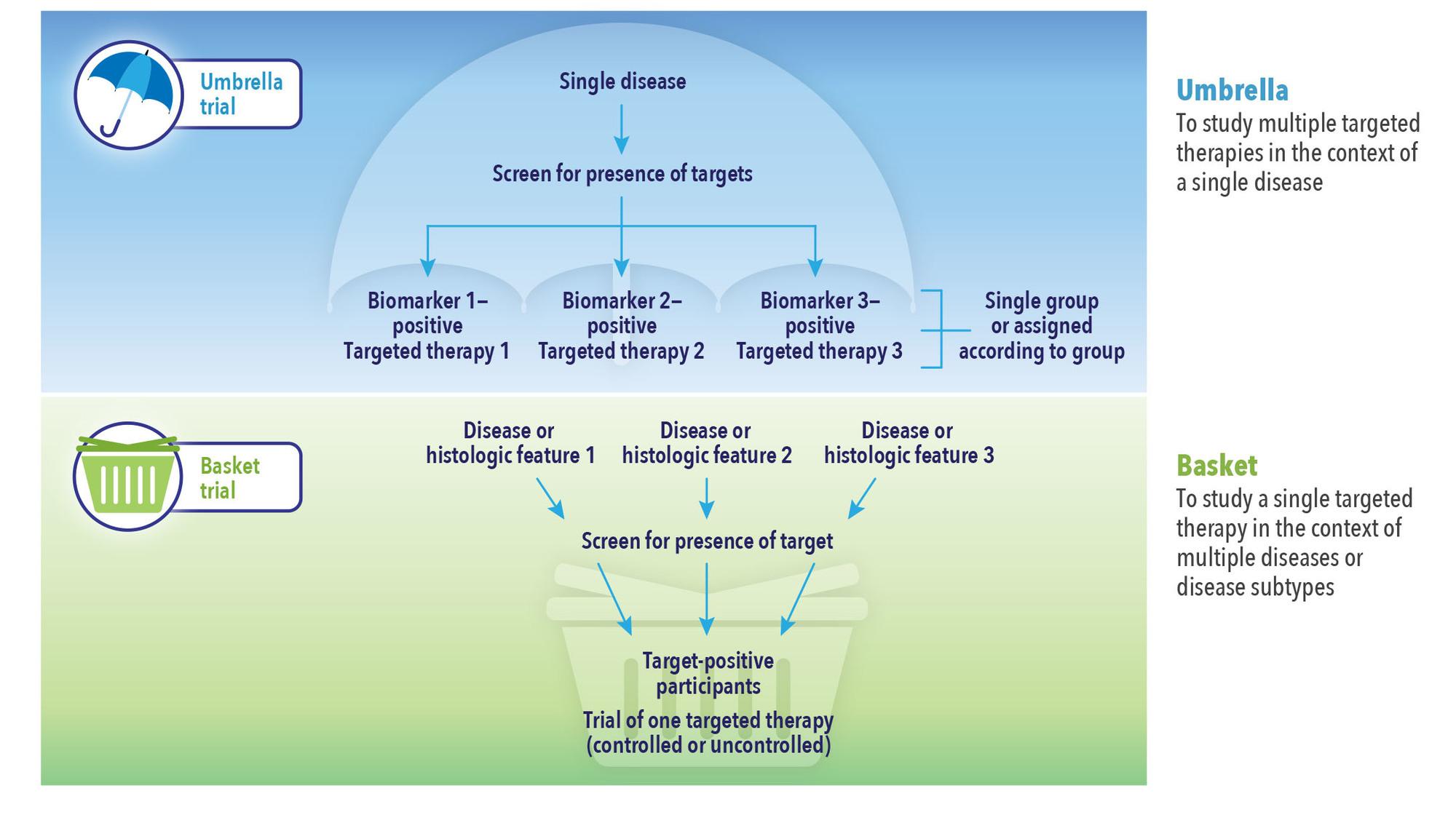
- Basket and umbrella trial designs, types of master protocols that may address multiple research questions under one protocol to identify target indications and patient populations
Adaptive vs. Traditional Designs
Traditional designs contribute to high failure rates and escalating costs. Traditionally, each trial is designed to answer only one narrow scientific question at a time in sequence. Moreover, answers to pivotal research questions are often obtained only at the end of the trial. In contrast, adaptive designs potentially allow a trial to answer multiple questions at once, leveraging accumulating data so early findings can inform decisions in an ongoing process.
Adaptive designs allow for prospectively planned modifications to one or more aspects of the design based on accumulating data from patients in the trial. Modifying trials as they progress can accelerate timelines, reduce costs, and generate more knowledge, thereby improving the overall quality and efficiency of decision making.
The adaptation process is typically prescribed in the trial protocol. Modifications may include dosage, sample size, patient selection criteria, and novel drug combinations, as well as specific indications as more information becomes available. The trial protocol is designed before the trial begins; the protocol pre-specifies the adaptation schedule and processes.
Surprisingly, innovative designs are not used as often as one would expect given that the methods are well established, more efficient, and regulators encourage their use. The largest untapped opportunities are arguably in the early phases of clinical development where adoption of innovative designs may, in fact, lead to an accelerated approval.
Regulatory Support and Buy-in
In 2007, the European Medicines Agency (EMA) began introducing frameworks for adaptive designs and encouraged their use.3 The FDA provided draft guidance in 2010, which was then refined and finalized in 2019.4,5 The FDA also drafted Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics in 2018.6 European Clinical Trial Facilitation Group (CTFG) perspectives on complex clinical trials with master protocols were presented in 2019.7 The International Council for Harmonisation (ICH) issued a final concept paper on adaptive clinical trials in 2019 with final
guidance expected in three years.8
Our experience in submitting protocols with adaptive designs in early stage oncology trials to United States and European regulators is that they not only accept these designs, they actively encourage them. Investigators have a growing understanding of the many benefits of these approaches and the world is now onboard with these study designs. Regulatory agencies not only promote usage, they welcome dialogue with sponsors pursuing these models. Early engagement with regulatory agencies is key. The FDA, for example, also strongly encourages sponsors to discuss plans to develop drugs under a master protocol with the clinical review division early in the program to obtain feedback.
Adaptive Design in Current Practice: Defining the Maximum Tolerated Dose
Accurately defining the optimal dose in oncology clinical trials is a common challenge. The correct maximum tolerated dose (MTD) estimation rate is only about 40%,9 which may result in patients being treated at subtherapeutic doses or doses that are too toxic. Selection of the wrong dose can not only disrupt the outcomes of all subsequent phases, it can, without a correction, ultimately lead to the development program’s failure. Most trials identify MTD using the 3+3 design, a rule-based design which offers simplicity, convenience, and familiarity. However, the 3+3 design method has limitations, including:
- It is defined based only on data from last dose
- The method ignores uncertainty
- There is no ability to re-escalate
- Cohort sizes are fixed
Because 3+3 offers a poor ability to determine the correct MTD, we do not recommend it. Several improved, rulebased, dose escalation designs are available, including the Modified Toxicity Probability Interval (mTPI) design and Bayesian Optimal INterval (BOIN) design. These designs offer more accuracy and flexibility than 3+3; however, they are not able to match the accuracy of a model-based design, therefore, we only recommend using these designs if the number of dose levels to be tested is fewer than five.
In most situations, the CRM design is the best choice for dose escalation studies. CRM is an adaptive, Bayesian, model-based approach that is superior to the 3+3 design. The CRM uses simulation software to efficiently evaluate a larger number of doses to estimate the MTD more precisely compared to 3+3, mTPI, and BOIN.10 CRM uses all data to update the estimation of the MTD and to allocate the next patients, either in cohorts or continuously. The model is frequently updated and thus is improved as data accrues, allowing researchers to make better, more efficient use of data. The model is typically updated within one working day.
The CRM provides an increased chance of treating study patients around the MTD and a decreased chance of exposing patients to dose levels greater than MTD.10 Also, in many cases the CRM can determine MTD from a smaller number of patients, which may lead to cost savings and a shortened timeline. CRM also provides more flexibility than a rule-based model, both scientifically and operationally. Rules are tailored for each study and expected toxicity profile, and priority can be given to MTD accuracy or study timelines.
Because of their flexibility, adaptive designs can overcome inherent limitations in the fixed structure of traditional designs. For example, CRM can adapt to accommodate late toxicities because it builds on previous knowledge, allowing it to generate predictions and directional guidance that can steer determination of the doses to investigate in combination trials. Borrowing of information also makes subsequent trials (e.g., defining other populations) more efficient.
Although the scientific methodology can be more difficult to understand, operational considerations for a CRM design resemble those of a 3+3 design. While a CRM design does require more front-end time, overall, it may save time by requiring fewer patients and by allowing for more rapid progression through early dosing levels depending on the operating characteristics and rules that are established in the design.
Looking at the big picture, from a cost perspective, a CRM design carries a much lower risk of overestimating or underestimating the MTD. In fact, considering the potential costs of taking a suboptimal dose into the next phase, it becomes clear that identifying the right MTD in the dose escalation phase could arguably generate the greatest cost savings, and advantage, that a program could gain.
Master Protocols: Efficient and Accelerated Development That Can Improve the Probability of Success
Master protocols employed in the early stages of a trial can help answer multiple questions simultaneously using a single infrastructure, design, and protocol, not only adding speed and efficiencies but rapid learning and data-driven, improved decision making (See Figure 1). Study teams can flex midstream, for example, to add or remove indication cohorts, drug combinations, and conduct other investigations in response to early findings without having to go back to the drawing board to write a new protocol and set up additional studies.
Master protocols may be used for exploratory purposes or in support of a marketing application. Many designs can be made adaptive, making them both more efficient and better able to answer questions accurately, and several innovations can be applied within a master protocol to improve trial efficiency. In an umbrella trial, for example, a common control arm can be used to reduce sample size. In a basket trial, a Bayesian hierarchical model could allow for information borrowing across patient cohorts and detect signals earlier with high efficiency.
In both umbrella and basket trials, investigators may be able to save resources and treat more patients with more promising drugs by adding or stopping indication cohorts and/or treatment arms, or they can adjust the randomization ratio among treatment arms based on interim analysis results. Bayesian decision rules based on posterior probability of meaningful treatment effect or success in future trials would provide flexibility in decision making and interim data monitoring, making it easier to detect efficacy signals earlier and reduce sample size.
Leveraging master protocols in the early stages of a trial can be particularly impactful to develop, amend, and answer hypotheses. Master protocols can provide multiple benefits:
- Increase speed and quality of decisions: de-risk by accelerating successful investigations and failing faster
- Reduce costs: shared trial infrastructure, design, and protocol deliver cost efficiencies; Deloitte estimates master protocols reduce costs by an estimated 13-18%12
- Shorten timeline: efficiencies accelerate effective therapies to market; Deloitte estimates master protocols reduce timelines by 12-15%12
- Benefit patients: patients are screened and allocated to the appropriate sub-study with the applicable treatments
Master protocol trials offer very practical operational benefits. For example, the use of a common protocol allows amendments to be developed, reviewed, and approved more quickly. Other efficiencies include site contracts and budget negotiations, streamlined site communications, and increased enrollment momentum. In balance, our experiences show the efficiencies and benefits far outweigh the complexities.
Trials that address many questions simultaneously using a master protocol can be operationally complicated. However, these complexities can be managed, even, for example, in complex global studies used to support a marketing application. Operational activities will not be linear, but instead are likely to run concurrently. Study teams must be poised to manage near-constant change, but with proper diligence and operational excellence, great advantages can be realized with these innovative designs.
References
- Pharma Intelligence Informa. Pharma R&D Annual Review 2020. March 2020. Available at: https://pharmaintelligence.informa.com/~/media/informa-shop-window/pharma/2020/files/whitepapers/rd-review-2020-whitepaper-update.pdf. Accessed July 22, 2020.
- Wong CH, Siah KW, Lo AW. Estimation of Clinical Trial Success Rates and Related Parameters. Biostatistics. 2019 Apr 1;20(2):273-286. doi: 10.1093/biostatistics/kxx069. Epub 2018 Jan 31.
- European Medicines Agency. Reflection Paper on Methodological Issues in Confirmatory Clinical Trials Planned With an Adaptive Design. 18 October 2007. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-methodological-issues-confirmatory-clinical-trials-planned-adaptive-design_en.pdf. Accessed July 22, 2020.
- US Food and Drug Administration (FDA). Draft Guidance for Industry on Adaptive Design Clinical Trials for Drugs and Biologics; Availability. 26 February 2010. Available at: https://www.federalregister.gov/documents/2010/02/26/2010-3980/draft-guidance-for-industry-on-adaptive-design-clinical-trials-for-drugs-and-biologics-availability. Accessed July 22, 2020.
- US Food and Drug Administration (FDA). Adaptive Designs for Clinical Trials of Drugs and Biologics. November 2019. Available at: https://www.fda.gov/media/78495/download. Accessed July 25, 2020.
- US Food and Drug Administration (FDA). Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics. September 2018. Available at: https://www.fda.gov/media/120721/download. Accessed July 25, 2020.
- Christensen D. European CTFG Perspective on: Complex Clinical Trials with Master Protocols. In: Clinical Trial Facilitation Group (CTFG); 2019.
- International Council for Harmonisation. Continued Advancement in Global Harmonisation Efforts as ICH Prepares for 30 Year Commemoration. 27 November 2019. Available at: https://admin.ich.org/sites/default/files/2019-11/ICH39Singapore_PressRelease_2019_1127_Final_0.pdf. Accessed September 30, 2020.
- North B, Kocher HM, Sasieni P. A New Pragmatic Design for Dose Escalation in Phase 1 Clinical Trials Using an Adaptive Continual Reassessment Method. BMC Cancer. 2019 Jun 26;19(1):632. doi: 10.1186/s12885-019-5801-3. Epub 2019 Jun 26.
- Wheeler GM, Mander AP, Bedding A, et al. How to Design a Dose-finding Study Using the Continual Reassessment Method. BMC Medical Research Methodology. 2019 Jan 18;19(1):18. doi: 10.1186/s12874-018-0638-z. Epub 2019 Jan 18.
- Woodcock J, LaVange LM. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. New England Journal of Medicine. 2017 Jul 6;377(1):62-70. doi: 10.1056/NEJMra1510062. Epub 2017 Jul 6.
- Deloitte Center for Health Solutions. Master Protocols: Shifting the Drug Development Paradigm. 2018. Available at: https://www2.deloitte.com/content/dam/insights/us/articles/4509_Master-protocols/DI_Master-protocols.pdf. Accessed October 16, 2020.
For more information, please contact
[email protected], [email protected], [email protected], [email protected], or [email protected].