FALL 2020, THE EVIDENCE FORUM, WHITE PAPER
 Andrew Bevan, MRes, CBiol, CSci, MRSB Senior Director Project Management Peri- and Post-Approval Studies Evidera, a PPD business |  Valeria Burrone, MEng Senior Project Manager Peri- and Post-Approval Studies Evidera, a PPD business |  Lorna Graham, MSc Director Project Management Peri- and Post-Approval Studies Evidera, a PPD business |
Pediatrics started to emerge as a medical specialty in the United States (US) with the founding of the American Pediatric Society in 1888. However, the field of pediatrics as we know it today originated with the establishment of the American Academy of Pediatrics and American Board of Pediatrics in the 1930s, which were set up to promote excellence in medical care for children and adolescents.
Physiological and psychological development is subject to continuous change from birth through adolescence, which means children should not be considered “small-scale” adults.1,2 However, as many as 54% of medicines prescribed to children in the US have not been tested for safety and efficacy in this population and are used as “off label” drugs.3 History has shown that children may be exposed to serious unintended harm if the efficacy and safety of medications is not adequately tested. Examples include gray baby syndrome, a type of circulatory collapse associated with chloramphenicol use in neonates; refractory hypotension and death associated with the use of verapamil for treatment of infants with supraventricular tachycardia; serious extrapyramidal dysfunction and bladder retention leading to hospitalization after treatment with domperidone, and many more.4-7 This does not mean that the extrapolation of data from studies performed in adult populations has no place in pediatric medicine. It can be beneficial when it is reasonable to assume that the course of disease progression and the response to treatment is similar in children compared to adults, thus minimizing the exposure of children to clinical trials and increasing the speed and efficacy of pediatric drug development giving pediatric patients access to safe and effective medicines more quickly. However, when it is not safe to make these assumptions it’s clear that specific pediatric trials are needed.
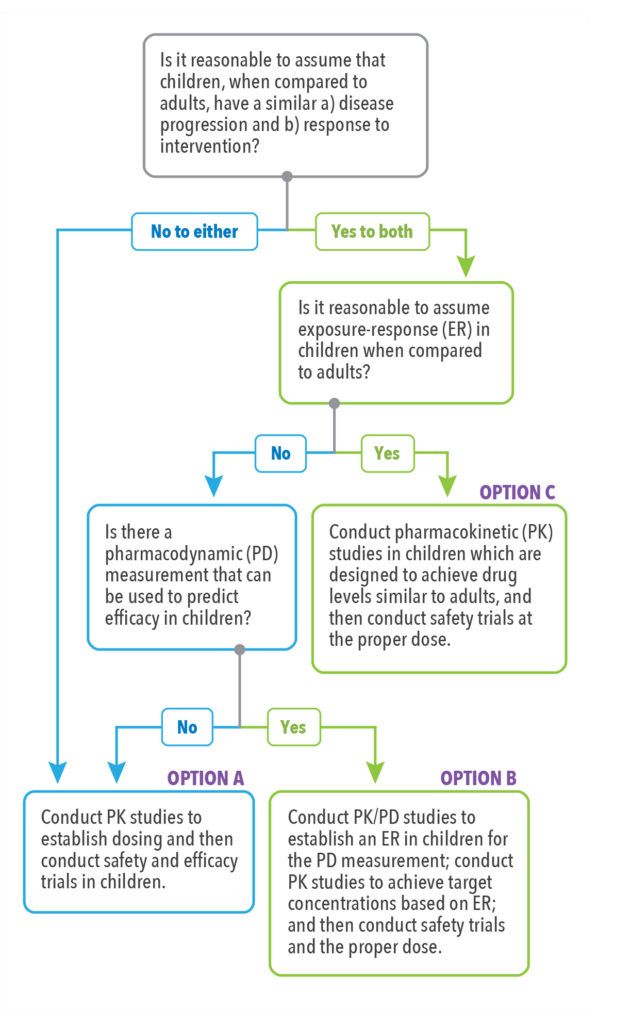
To facilitate the decision-making process, the U.S. Food and Drug Administration (FDA) developed the FDA Pediatric Study Decision Tree (See Figure 1),8 a simple assumptions-based framework that can be a helpful starting point in determining the pediatric studies (excluding oncology studies) necessary for labeling based on the ability to extrapolate efficacy from adult populations or other data.
In this article, we discuss the impact of regulatory changes governing pediatric drug development and pediatric drug labeling, some of the challenges of performing clinical trials in pediatric populations, and strategies that may be employed to help address these challenges.
US Pediatric Drug Legislation and Impact on Pediatric Drug Labeling
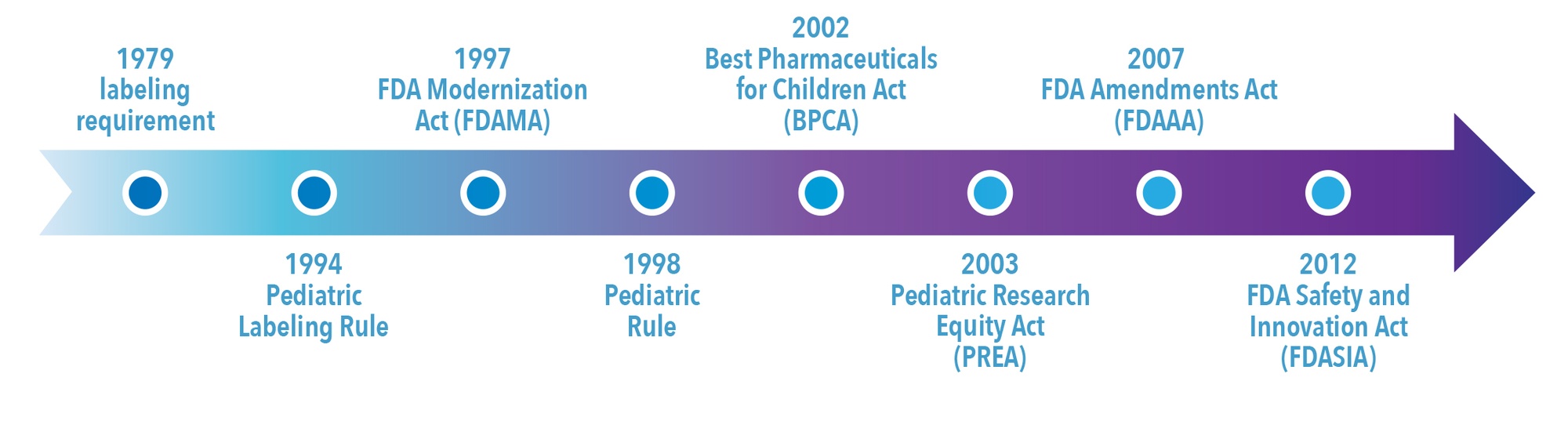
The major milestones in US pediatric drug legislation are shown in Figure 2. Legal measures to protect children from harmful medications were introduced in the US in the early part of the 20th century in response to fatalities due to medicinal products.9,10 However, it wasn’t until 1979 that the first notable FDA legal provision relating to pediatric drug labeling appeared. At that time the FDA issued a requirement for sponsors to conduct pediatric clinical trials before including pediatric information in the drug’s label. Even so, the FDA did not issue a final Pediatric Labeling Rule until 1994. This labeling rule introduced the extrapolation of adult data to children and required manufacturers of marketed drugs to evaluate whether data existed to support pediatric labeling supplements. However, it did not require companies to conduct pediatric trials, and the legislation proved to be relatively ineffective in improving pediatric use information.11
In 1997, the FDA created an incentive for companies to test drugs in pediatric populations with the Food and Drug Administration Modernization Act (FDAMA), which gave manufacturers an additional six months marketing exclusivity if they performed studies in children. This was voluntary. The 1998 Pediatric Rule gave the FDA the power to mandate that companies conduct pediatric studies for marketed new drugs and biologics and established the premise that unapproved products must be studied in pediatric populations. However, manufacturers could request, and be granted, waivers from these requirements if the product 1) did not represent a meaningful therapeutic benefit over existing treatments for pediatric patients and 2) was not likely to be used in a substantial number of pediatric patients. Therefore, the Pediatric Rule did not fundamentally increase the number of products with adequate pediatric labeling.
The Pediatric Rule was declared invalid by a federal court in 2002 on the basis that Congress had not given authority to the FDA to require extensive testing of drugs for children.12 In January 2002, the Best Pharmaceuticals for Children Act (BPCA) was passed. It renewed the six months of marketing and patent protection incentive introduced under the FDAMA to sponsors who voluntarily complete pediatric clinical studies outlined in a Written Request (i.e., a formal FDA request that studies be done in pediatric patients). In addition, the BPCA created the Office of Pediatric Therapeutics within the FDA and directed the National Institutes of Health (NIH) to establish a program for pediatric drug development for off-patent drugs. Under the BPCA, the FDA can issue a Written Request for pediatric studies in any indication and may expand indications for drug use, including orphan indications.
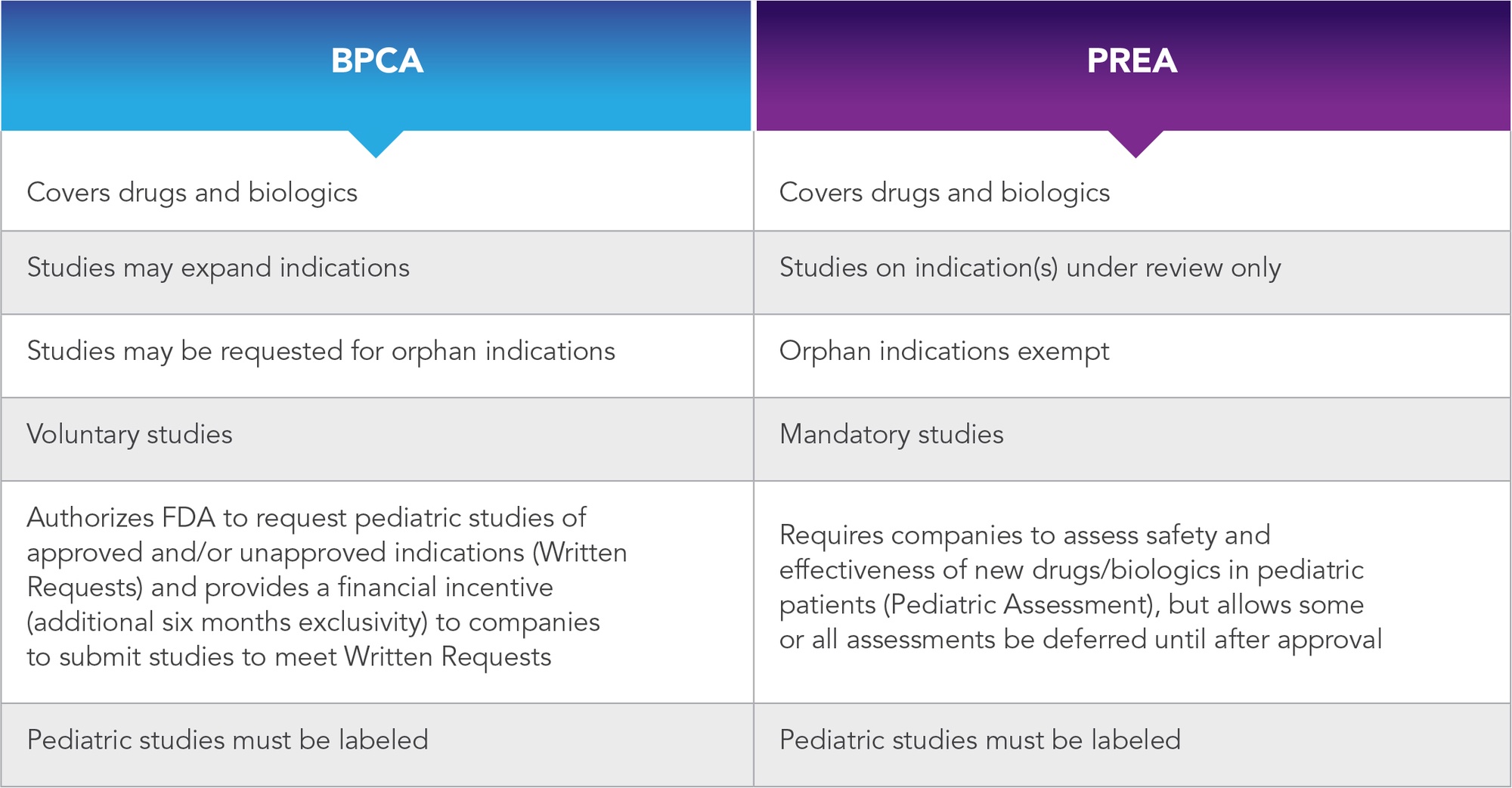
In addition to the voluntary incentives offered by BPCA, the Pediatric Research Equity Act (PREA) of 2003 amended the Federal Food, Drug, and Cosmetic Act. This gave the FDA the power to require companies to perform pediatric studies for products submitted in a new drug application (NDA) if the FDA determined that it is probable the product will be used to treat a sizeable number of pediatric patients, or if it will offer meaningful advancements over current therapies. Unlike the BPCA, the PREA limits the FDA to mandating studies on indication(s) contained in NDA submission(s), and therefore it cannot be used to expand indications. A comparison of the key features of the BPCA and the PREA is presented in Table 1.
Table 1. Comparison of PREA vs. BPCA
(adapted from https://www.fda.gov/media/91673/download)
In 2007, the BPCA and PREA were reauthorized for another five years under the Food and Drug Administration Amendments Act (FDAAA). The FDAAA also required that Written Requests, pediatric plans, deferrals, and waivers for the performance of studies in pediatric populations be reviewed by the FDA’s Pediatric Advisory Committee (PAC) and mandated that the results of pediatric studies be included in the product label even if they are negative or inconclusive. In 2012, the BPCA, PREA and the FDA PAC were made permanent under the Food and Drug Administration Safety and Innovation Act (FDASIA).
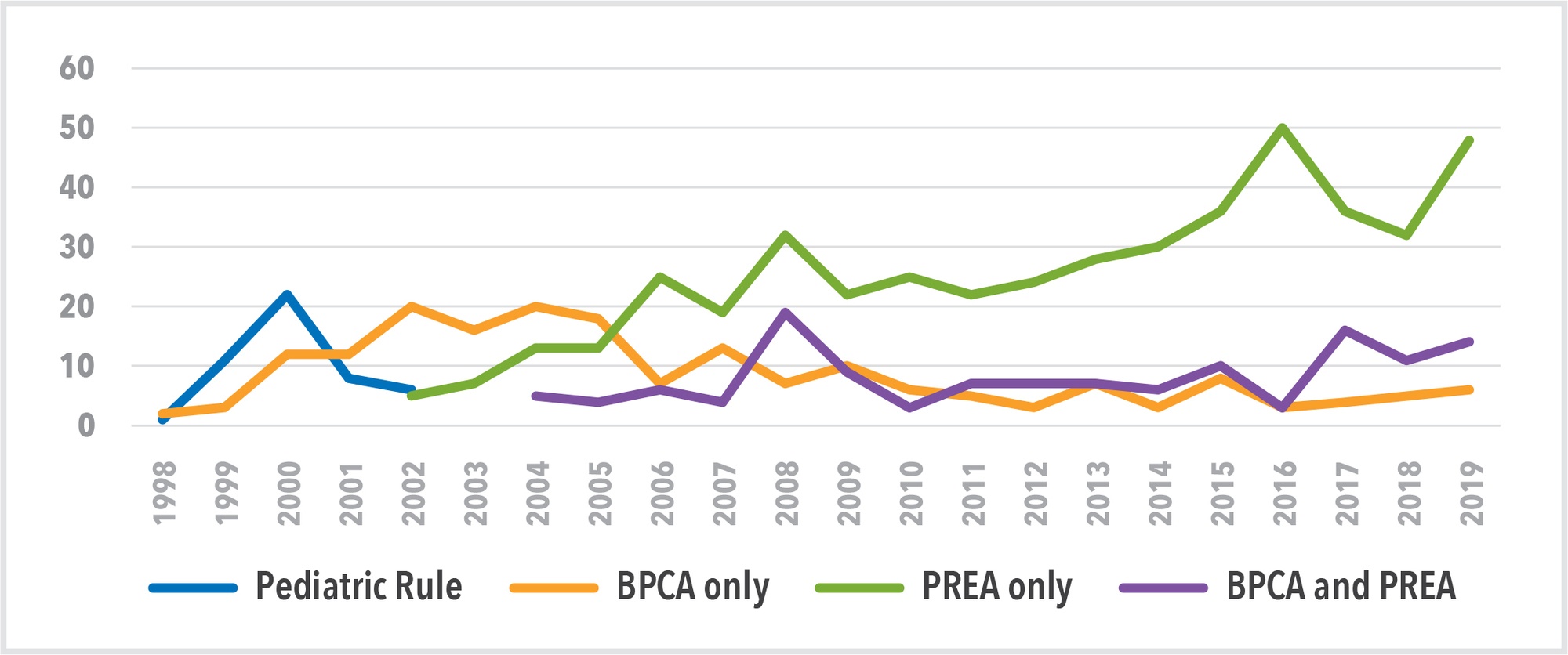
We can see the impact of the various legislative instruments (Pediatric Rule, BPCA and PREA) in Figure 3 which shows data from the FDA’s New Pediatric Labeling Information Database.13 The database provides details of 854 FDA pediatric labeling approvals over the last 22 years. This demonstrates that most label approvals—475—have been the result of enforcement under the PREA. Only 199 approvals are the result of incentivization under the BPCA alone, indicating that the stick has been stronger than the carrot in driving pediatric drug development in the US.
Figure 3. Impact of Legislative Instruments on FDA Pediatric Labeling Approvals
The Challenges of Conducting Pediatric Studies
Pediatric drug development is challenging and the financial incentives for companies to develop treatments for children can be low compared to the research and development (R&D) investment required. This may explain why the voluntary provisions under BPCA have not been more successful in driving pediatric labeling.
The first challenge of pediatric research is to define and identify the needs of the study population. The Center for Drug Evaluation and Research (CDER) generally divides the pediatric population into four groups: neonates (birth up to 1 month), infants (1 month up to 2 years), children (2 to 12 years), and adolescents (12 to 16 years).14 However, the pediatric population represents an extremely broad maturational range both physiologically and psychologically. As such, the conditions that affect this population and the factors that influence drug pharmacokinetics and pharmacodynamics are highly varied. Therefore, this somewhat arbitrary division is often an oversimplification. For some drugs being developed for pediatric populations it may be necessary to consider further subgroups based on maturity. For example, gastric pH can affect drug absorption. In neonates, gastric pH is close to neutral for the first 1 to 2 weeks of life and gradually declines until age 2 when it approaches adult levels.15 This means the relatively alkaline environment of the neonate or infant gut can result in ionization of weakly acidic drug molecules such as phenytoin, which is used to treat epilepsy and is best absorbed in its nonionized form. This reduces the bioavailability of the drug and therapeutic effect. Furthermore, renal excretion of drugs can also be reduced in neonates due to immature glomerular filtration, tubular secretion, and reabsorption leading to higher bioavailability and the potential for adverse reactions.15
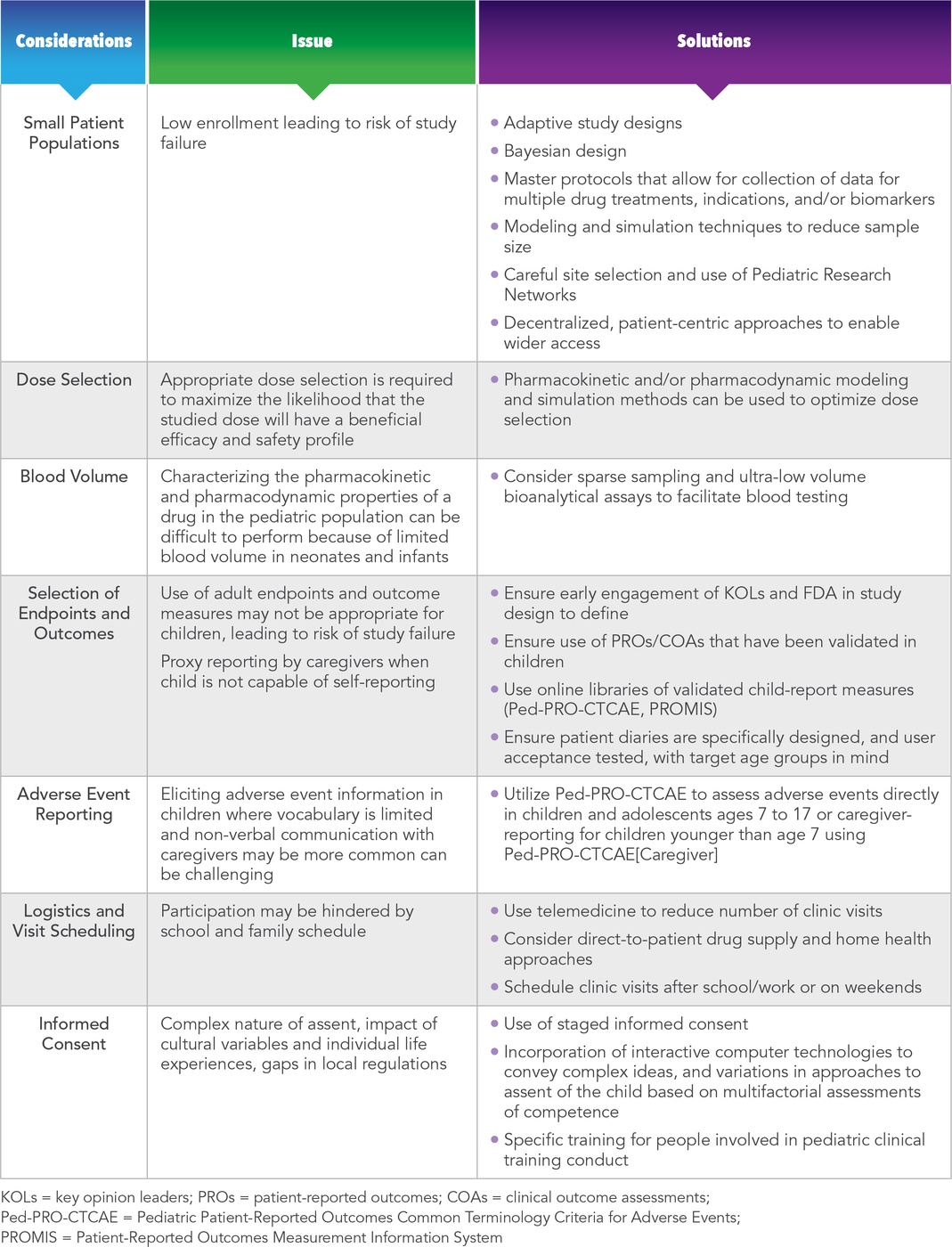
Other challenges often associated with performing pediatric studies include:
- Smaller disease populations compared to adults (e.g., type 2 diabetes)
- Selection of appropriate dose levels in children
- Blood volume and tissue sampling restrictions
- Availability of validated clinical endpoints in the age groups under study (e.g., lack of validated pediatric patient-reported outcomes (PROs) for indications such as asthma, diabetes, irritable bowel syndrome, oncology)
- Getting accurate adverse event information from infants and young children
- The impact of school and family life on study logistics and visit scheduling
- Obtaining informed consent and assent
These challenges can lead to potential inconsistencies in the types of outcome information contained in the labeling for drugs approved for the same indication.15 The impact of these complexities on the time, cost, and quality of development programs in pediatrics can be significant in the absence of the appropriate expertise to proactively identify issues and plan mitigation strategies. The issues surrounding these challenges and potential solutions to help address them are summarized in Table 2.
Conclusion
Voluntary incentivization by the FDA in the form of extended exclusivity and patient protection has resulted in an increase in the number of products that are approved for use in pediatrics in the last 20 years. However, the main driver for pediatric label approvals has been the PREA, which authorizes the FDA to impose a requirement on companies to perform pediatric studies. Performing clinical trials in pediatric populations can be challenging, and the additional R&D investment and expertise needed to achieve success can deter sponsors from seeking pediatric labels for their products. Identifying these challenges and the strategies that can be employed to help overcome them is an important step towards achieving success.
References
- Ferro A. Paediatric Prescribing: Why Children Are Not Small Adults. Br J Clin Pharmacol. 2015 Mar;79(3):351-3. doi: 10.1111/bcp.12540.
- Larcher V. Children Are Not Small Adults: Significance of Biological and Cognitive Development in Medical Practice. In: Schramme T, Edwards S. eds. Handbook of the Philosophy of Medicine. Crown Copyright 2015. Available at: https://link.springer.com/content/pdf/10.1007%2F978-94-017-8706-2_16-1.pdf. Accessed September 18, 2020.
- Yackey K, Stukus K, Cohen D, Kline D, Zhao S, Stanley R. Off-label Medication Prescribing Patterns in Pediatrics: An Update. Hosp Pediatr. 2019 Mar;9(3):186-193. doi: 10.1542/hpeds.2018-0168. Epub 2019 Feb 11.
- Klassen TP, Hartling L, Craig JC, Offringa M. Children Are Not Just Small Adults: The Urgent Need for High-Quality Trial Evidence in Children. PLoS Med. 2008 Aug 12;5(8): e172. doi: 10.1371/journal.pmed.0050172.
- Caldwell PHY, Murphy SB, Butow PN, Craig JC. Clinical Trials in Children. Lancet. 2004 Aug 28-Sep 3;364(9436):803-11. doi: 10.1016/S0140-6736(04)16942-0.
- Johnson TN. The Development of Drug Metabolising Enzymes and Their Influence on the Susceptibility to Adverse Drug Reactions in Children. Toxicology. 2003 Oct 1;192(1):37-48. doi: 10.1016/s0300-483x(03)00249-x.
- Garson A Jr. Medicolegal Problems in the Management of Cardiac Arrhythmias in Children. Pediatrics. 1987 Jan;79(1):84-8.
- US Food & Drug Administration (FDA). Pediatric Science and Research Activities. Available at: https://www.fda.gov/science-research/pediatrics/pediatric-science-and-research-activities. Accessed September 17, 2020.
- DeHovitz RE. The 1901 St Louis Incident: The First Modern Medical Disaster. Pediatrics. 2014 Jun;133(6):964-5. doi: 10.1542/peds.2013-2817.
- US Food & Drug Administration (FDA). Ballentine C. Sulfanilamide Disaster. FDA Consumer Magazine. 1981 June. Available at: https://www.fda.gov/files/about%20fda/published/The-Sulfanilamide-Disaster.pdf. Accessed September 18, 2020.
- Bucci-Rechtweg C. Enhancing the Pediatric Drug Development Framework to Deliver Better Pediatric Therapies Tomorrow. Clin Ther. 2017 Oct;39(10):1920-1932. doi: 10.1016/j.clinthera.2017.07.043. Epub 2017 Aug 14.
- The Climate Change and Public Health Law Site. FDA Does Not Have the Authority to Require Pediatric Testing of Drugs – Association of American Physicians and Surgeons, Inc. v. US Food and Drug Admin., 226 F.Supp.2d 204 (DDC Oct 17, 2002). Available at: https://biotech.law.lsu.edu/cases/FDA/aaps_v_fda.htm. Accessed September 17, 2020.
- US Food & Drug Administration (FDA). New Pediatric Labeling Information Database. Available at: https://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase&displayAll=true. Accessed September 17, 2020.
- US Food & Drug Administration (FDA). General Clinical Pharmacology Considerations for Pediatric Studies for Drugs and Biological Products – Guidance for Industry. Draft Guidance. 2014 Dec. Available at: https://www.fda.gov/media/90358/download. Accessed September 17, 2020.
- Lu H, Rosenbaum S. Developmental Pharmacokinetics in Pediatric Populations. J Pediatr Pharmacol Ther. 2014 Oct-Dec; 19(4): 262–276. doi: 10.5863/1551-6776-19.4.262.
For more information, please contact
[email protected], [email protected], or [email protected].