SPRING 2019, THE EVIDENCE FORUM, WHITE PAPER
 Katie Gardner, PhD Senior Director Evidence, Modeling & Communication; Executive Director Center of Excellence for Market Access Evidera |  Matthew Bending, PhD Senior Principal and Executive Director of HTA Strategy Market Access Consulting Evidera |
Almost 20 years ago the European Parliament adopted legislation in order to incentivise manufacturers to develop and market medicinal products to treat rare diseases.1 Although this legislation stimulated research and development in the European Union (EU), access to these treatments remains inconsistent among EU Member States, which may in part be due to differences in assessment criteria between the various stakeholders.2,3 There is often a gap between regulatory, health technology assessment (HTA), and payer requirements, which is further complicated by inconsistencies in value assessment between individual EU Member States.
Given the low patient numbers and the high unmet need for new treatments in rare diseases, it is crucial for manufacturers to develop an evidence package that meets the needs of all stakeholders – including regulators, HTA bodies, payers, and patients – to achieve access with certainty and speed across the EU. However, assessment and appraisal of medicinal products to treat rare diseases can be challenging in Europe given the differences in evidence requirements and assessment methods, which often results in different stakeholders reaching different conclusions on the access and reimbursement of a product.
Over the past several years we have seen an increase in collaboration between regulatory and HTA bodies as well as increased collaboration among country authorities. The ability for manufacturers to participate in integrated Early Scientific Advice (ESA) and collaborative multi-country HTA initiatives, such as the European Network for Health Technology Assessment (EUnetHTA), can help manufacturers of medicinal products for rare diseases optimise their product development and increase the likelihood of obtaining patient access across the EU.
Integrated Early Scientific Advice in Europe
There are several types of ESA available to manufacturers in Europe including regulatory-only advice (either with country-level agencies or the European Medicines Agency [EMA]), HTA-only advice (either with individual country-level agencies or multi-country collaborations), as well as multi-stakeholder integrated joint scientific advice which involves both regulatory and HTA (See Figure 1).
Figure 1. Types of Early Scientific Advice Available in Europe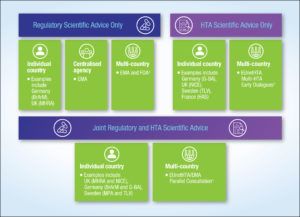
a. European Medicines Agency. Recent Update of the Guidance for Parallel EMA/FDA Scientific Advice, 15 Nov 2017. Available at: https://www.ema.europa.eu/documents/presentation/presenatation-recent-update-guidance-parallel-ema/fda-scientific-advice-t-vetter-ema_en.pdf. Accessed March 7, 2019
b. EUnetHTA. Procedure Description for EUnetHTA Multi-HTA Early Dialogues for Pharmaceuticals, 26 February 2018. Available at: https://www.eunethta.eu/wp-content/uploads/2018/03/Multi-HTA-Pharma-Procedure_180227.pdf. Accessed March 7, 2019
c. EUnetHTA and the European Medicines Agency. Guidance for Parallel Consultation (EMA/410962/2017), 30 June 2017. Available at: https://www.eunethta.eu/wp-content/uploads/2018/03/Guidance-on-Parallel-Consultation.pdf. Accessed March 7, 2019
Integrated joint ESA may be of particular utility in rare diseases since it provides manufacturers with an understanding of key evidence gaps and, importantly, allows them to explore how these gaps could be filled to meet both regulatory and HTA requirements.
The EMA/EUnetHTA parallel consultation brings together stakeholders from multiple countries, including both regulatory and HTA bodies. EMA/EUnetHTA parallel consultation includes two pathways – a consolidated pathway and an individual pathway.4 The consolidated pathway guarantees the input of the EUnetHTA Early Dialogues Working Party (EDWP) which includes HTA representatives from France (HAS), Germany (GBA), England (NICE), Italy (AIFA with alternate RER), Hungary (NIPN), and a shared seat for the Netherlands/Belgium (ZIN/RIZIV-INAMI), plus up to three additional HTA bodies. EDWP members are guaranteed to take part in the consultation. Only certain products can be selected for the consolidated parallel consultation pathway due to resource constraints, and the individual parallel consultation pathway is offered as an alternative.
To be considered for the consolidated parallel consultation pathway, a product must meet all the following criteria:
- 1. Includes a new mode of action for the indication
- 2. Targets a life-threatening or chronically debilitating disease
- 3. Responds to unmet need (no treatment or only unsatisfactory treatment available)
Patient engagement has been recognised to be an integral part of the EMA/EUnetHTA parallel consultation process, and individual patient experts have been welcomed to participate in consultations to date. Patients have been identified through patient organisations under the EMA framework for interaction and are invited to join all meetings.5 In addition, KOLs are invited to participate in the consolidated parallel consultation.
Obtaining scientific advice from both regulatory and HTA bodies across several countries in an integrated manner provides manufacturers with a strong rationale for clinical development plan decisions. However, there are often challenges to consider when seeking ESA, such as the intense and sometimes conflicting demands during product development, the need to gather internal cross-discipline alignment, and the risk of vastly different opinions from HTA bodies and regulators. It is important for manufacturers to identify the challenges early and seek support where necessary in order to maximise the potential of ESA.
Collaborative Multi-Country HTA Initiatives in Europe
One of the greatest challenges in rare diseases is the demonstration of clinical relative effectiveness, which is critical to HTA. Differences in HTA processes and methodologies can lead to differences in how clinical relative effectiveness is determined in assessments; collaboration by HTA bodies may decrease the disparity on how clinical relative effectiveness is both defined and assessed. EUnetHTA was set up to support collaboration between European HTA organisations and consists of a network of over 80 organisations in 30 European countries.6 EUnetHTA has developed methodological frameworks for collaborative production and sharing of HTA information and also undertakes assessments. However, participation by EU Member States in EUnetHTA is voluntary and funding is short-term. This means clinical assessments of the same technologies are still being conducted in parallel across Member States and there is no guarantee for the continuation of these voluntary HTA collaborations in the long term. A 2018 EU HTA Directive (2018/0018) published January 2018 and adopted April 2018 aimed to address some of these shortcomings.7 The Directive set out four pillars of work including joint clinical assessment (JCA) for all EMA approved pharmaceuticals. The JCA will focus on the comparative clinical assessment of the technology with clinical relative effectiveness a key domain. The JCA process will therefore require agreement from stakeholders on the centralised methods and assessment criteria used to determine clinical relative effectiveness.
The EU HTA Directive also aims to create synergies between the regulatory and HTA processes through mutual information sharing and better alignment of the timing between the proposed JCAs and the centralised marketing authorisation. Another pillar of work set out in the Directive is Joint Scientific Consultation (JSC), under which manufacturers can make a request for an early dialogue during the development phase of a product. These consultations can include only HTAs or EMA and HTAs in parallel. The aim of these consultations would be for manufacturers to seek advice on the data likely to be required for a potential future JCA. During the European Parliament’s September 2018 meeting it was agreed the JSC, when addressing orphan medicinal products (i.e., treatments for rare diseases that have been granted orphan drug status by the EMA), has to ensure that any new approach should not result in unnecessary delays for the product’s assessment compared to the current situation and should take into account the pragmatic approach undergone by EUnetHTA. In addition, it was agreed that a tailored clinical assessment pathway may be developed for orphan medicinal products due to the limited number of patients enrolled in clinical trials and/or the lack of a comparator.
The new EU HTA Directive has been welcomed by EURORDIS-Rare Diseases Europe, a non-governmental patient-driven alliance of more than 660 rare disease patient organisations across the 28 EU Member States, who stated that the Directive “introduces fairness, equity, high scientific standards and efficiency in the decision-making process” which is in the interest of people living with a rare disease and the wider patient community.8 EURORDIS-Rare Diseases Europe has also welcomed strategies to establish synergies between the EMA and EU HTA bodies emphasising the importance of quality of life as a key domain in both regulatory and HTA decision making.9
Currently there remain uncertainties surrounding the EU HTA assessment methods and their definitions, how the comparators will be selected, and what will be the role of real-world evidence and patient-reported outcomes. Some clarity on the methods for JCA was provided at the European Parliament’s September 2018 meeting, which highlighted the merit of EUnetHTA methods. During the meeting it was also agreed that the development of the rules of engagement for Joint EU HTA should consider the results of work already undertaken in the EUnetHTA Joint Actions, including methodological guidelines and evidence submission templates.
Outlook
The role the National Institute for Health and Clinical Excellence (NICE), which has been a leading participant in the EMA/EUnetHTA parallel consultation and EU HTA harmonisation initiatives, will play following the UK’s exit of the EU remains unclear. However, on 6 February NICE announced that it will collaborate with the Canadian Agency for Drugs and Technology in Health (CADTH) to offer parallel scientific advice which demonstrates the agency’s willingness to expand its partnerships with decision-making bodies beyond Europe.10
There are also examples of collaborations between EU Member States that extend beyond the clinical assessment and include pricing and reimbursement decisions. The Beneluxa Initiative comprises the Netherlands, Belgium, Luxembourg, Austria, and Ireland and aims to “ensure sustainable access to innovative medicine at affordable cost for our patients.”11 The initiative includes a collaborative approach to focus on evaluating high-cost orphan medicines and involves both joint HTA procedures and collaboration on price negotiations.
The aim of multi-stakeholder collaborations should be to streamline and clarify evidence requirements to increase transparency, consistency, and timeliness. The goal of any harmonisation initiative should be to increase the speed and breadth of access of new medicines for patients. Whether the convergence of clinical assessment makes the hurdle for access to medicines to treat rare diseases higher remains to be seen; however, it may provide a clearer signal of the evidence requirements needed to meet European stakeholder needs, thus decreasing the likelihood of unexpected missteps along the way. In rare diseases, where small patient populations and limited resources mean gathering evidence is a significant challenge, anything to smooth the path to patient access is welcome.
References
- Publications Office of the EU. Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on Orphan Medicinal Products. Available at: https://publications.europa.eu/en/publication-detail/-/publication/d845e34f-2cd3-4217-9ab3-1ccfb1bb3be3/language-en. Accessed February 12, 2019.
- Szegedi M, Zelei T, Arickx F, et al. The European Challenges of Funding Orphan Medicinal Products. Orphanet J Rare Dis. 2018 Nov 6;13(1):184. doi: 10.1186/s13023-018-0927-y.
- Malinowski KP, Kawalec P, Trabka W, Sowada C, Pilc A. Reimbursement of Orphan Drugs in Europe in Relation to the Type of Authorization by the European Medicines Agency and the Decision Making Based on Health Technology Assessment. Front Pharmacol. 2018 Nov 12;9:1263. doi: 10.3389/fphar.2018.01263. eCollection 2018.
- European Medicines Agency. EUnetHTA. Guidance for Parallel Consultation. June 30, 2017. Available at: https://www.ema.europa.eu/documents/regulatory-procedural-guideline/guidance-parallel-consultation_en.pdf. Accessed February 12, 2019.
- European Medicines Agency. Moseley J. The First Experience with the Newly Launched Parallel Consultation Platform. November 23, 2017. Available at: https://www.ema.europa.eu/en/documents/presentation/presenatation-first-experience-newly-launched-parallel-consultation-platform-j-moseley-ema_en.pdf. Accessed February 12, 2019.
- EUnetHTA. Available at: https://www.eunethta.eu/. Accessed February 12, 2019.
- European Commission. Proposal for a Regulation of the European Parliament and of the Council on Health Technology Assessment and Amending Directive 2011/24/EU. January 31, 2018. Available at: https://ec.europa.eu/health/sites/health/files/technology_assessment/docs/com2018_51final_en.pdf. Accessed February 15, 2019.
- EURORDIS Rare Diseases Europe. Why EURORDIS Supports the Proposal for a Regulation on Health Technology Assessment (HTA) Cooperation in Europe. Available at: http://download2.eurordis.org.s3.amazonaws.com/positionpapers/Statement_final.pdf. Accessed February 12, 2019.
- EUnetHTA Magazine. Expanding Dialogue between HTA Bodies and Stakeholders. Fall Issue 2018. Available at: https://www.linkedin.com/pulse/expanding-dialogue-between-hta-bodies-stakeholders-eunethta–1f. Accessed February 12, 2019.
- NICE National Institute for Health and Care Excellence. NICE Collaborates with the Canadian Agency for Drugs and Technology in Health to Offer Parallel Scientific Advice. February 6, 2019. Available at: https://www.nice.org.uk/news/article/nice-collaborates-with-the-canadian-agency-for-drugs-and-technology-in-health-to-offer-parallel-scientific-advice. Accessed February 12, 2019.
- Beneluxa | Initiative on Pharmaceutical Policy. Available at: http://www.beneluxa.org/. Accessed February 12, 2019.
For more information, please contact
[email protected] or [email protected]