SPRING 2019, THE EVIDENCE FORUM, WHITE PAPER
 Andrea Schmetz, MBA Consultant Market Access Consulting Evidera |  Helena Emich, PhD Senior Market Access Writer Evidence Synthesis, Modeling & Communication Evidera |  Krista Payne, MEd Vice President General Manager Real-World Evidence Evidera |  Delphine Saragoussi, MD, MScPH Research Scientist Real-World Evidence Evidera |
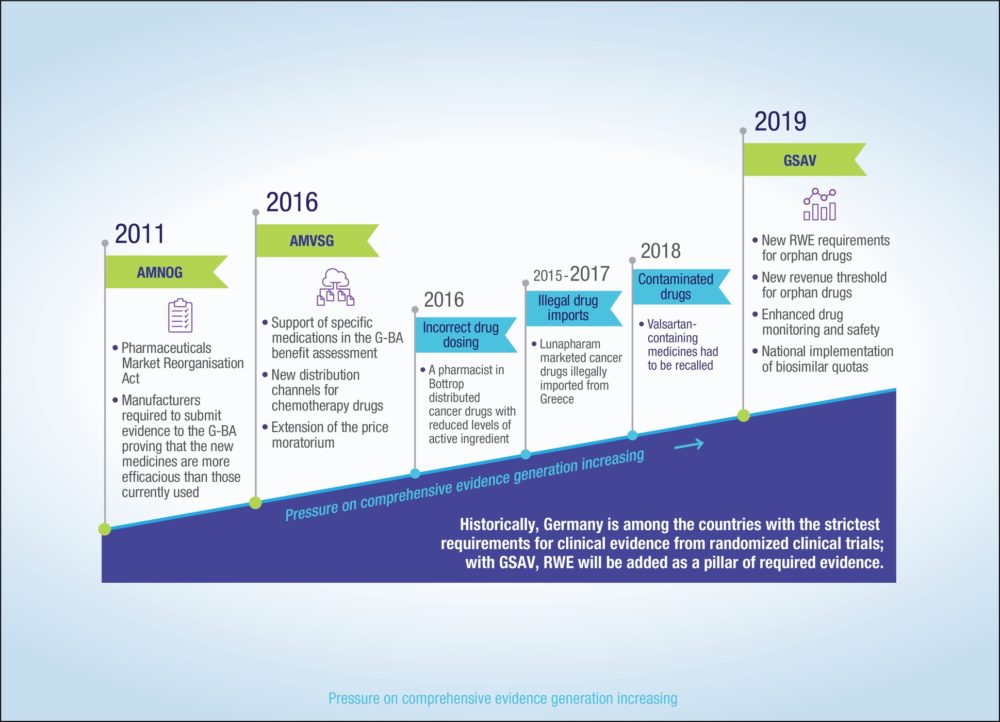
As a reaction to the recent drug safety incidences in Germany (including drugs contaminated with potentially carcinogenic substances, illegal drug imports, and incorrect drug dosing by a pharmacist), the German Ministry of Health introduced a draft “law for more safety in the supply of pharmaceuticals” (GSAV) in November 2018 (See Figure 1). The bill is still to be voted on in both chambers of the German Parliament and, if accepted, will come into effect 1 July 2019.
One of the main components of the draft law is the demand for better evidence for orphan drugs and drugs with conditional approval and a change to the revenue threshold for orphan drugs.
In general, orphan drugs brought to market in Germany enjoy the advantage of an assumed benefit, meaning the worst potential outcome in health technology assessment (HTA) is a non-quantifiable benefit. However, traditionally, if the product’s revenue in the retail market exceeds an annual turnover threshold of €50 million, the product then requires a full benefit assessment under AMNOG without the orphan medicine advantage of an assumed benefit. In this case, the manufacturer needs to provide additional evidence, likely against an active comparator. Beginning in July, all revenue from product sales – not only retail but also hospital, etc., – will go towards the €50 million threshold. This could affect several drugs and give the G-BA the legal means to reassess several orphan assets, closing an obvious accounting loophole.
In the light of recent concerns with safety and long-term efficacy of multiple orphan drugs, the Ministry of Health has also taken steps to increase evidence requirements for orphan drugs. The G-BA has already, within current means, tried to limit the long-term impact of insufficient data with additional restrictions, e.g., in the case of a treatment for primary biliary sclerosis assessed in 2017, where the G-BA demanded a re-evaluation with long-term data to be made available in 2023. Similarly, a treatment for spinal muscular atrophy (SMA) was also given restrictions mandating additional evidence, albeit the timeline is only until 2020 when the drug is due to be reassessed. The new GSAV law will expand the means to increase restrictions even more, as it specifies that the G-BA can demand additional evidence for all assets with conditional approval (given due to missing evidence) or for orphan drugs (where the evidence base is considered low). This additional data required should be collected as real-world evidence (RWE), the exact specifications to be outlined by the G-BA. The evidence will be reviewed at least yearly, making it technically possible for the G-BA to re-evaluate drugs on a yearly basis. Should the manufacturer fail to comply with the request for additional evidence or if the submitted evidence leads to a negative reassessment outcome, the German statutory health insurance system (GKV) will have the right to discount the price of the asset. Of note, a lack of evidence can lead to a price discount, however, favourable evidence cannot lead to a price increase due to the existing price moratorium, which is in force until the end of 2022.
The new regulation regarding additional evidence presents new opportunities to incorporate real-world evidence, which so far has not been looked upon as acceptable by the German HTA. Considering the impact of this data, any future evidence generated should be both comprehensive and high quality.
Early engagement in strategic real-world evidence generation planning, in the context of these new opportunities for market access optimization, will be paramount ...
It is possible that in the future this new rule could be expanded to all drugs assessed under AMNOG, Germany’s law regarding the marketing of pharmaceutical products. This would offer manufacturers the ability to launch a new product earlier based on limited evidence and substantiating the evidence base with RWE data in the years after launch, as long as manufacturers are confident that the evidence produced will be able to support their needs. The RWE data generated in Germany (the largest market in Europe) could then potentially be used to support launches in other European markets as well. Along these lines, consideration should be given to whether there are potential synergies between post-authorisation safety studies requested by the European Medicines Agency (EMA) and RWE requested by Germany.
Real-world evidence methodologies include a diverse array of study types depending on the questions that need to be answered and the data needed to answer them appropriately.
For example:
- Early natural history studies are used to characterize patient groups of interest and unmet medical need, and with the growing attention on rare diseases, there is a greater need to accurately define the profile, characteristics, and disease outcomes of target patient populations. (See “Natural History Studies in Rare Diseases and Genetic Biomarkers” by Bevan, Ringo, Fitzgerald, Kearney, and Saragoussi in this issue of The Evidence Forum.)
- Burden of illness studies evaluate patterns and costs of care and provide insight into the journey of rare disease patients, including the economic challenges, the emotional burden, and the effects on their quality of life. These data can be particularly revealing of those sufferings from rare diseases, where care options are limited and often difficult to access and the emotional burden can be overwhelming with little support from others with the same condition. Data from these studies can help provide a more complete and compelling value story for rare disease treatments.
- Comparative effectiveness studies help identify the value of a new treatment compared to existing treatments and can guide decisions on additional real-world studies that may be needed to show additional benefits. These studies can be especially beneficial in rare disease treatments where standard of care is often inadequate.
- Disease and treatment/product registries can be very useful in gathering data on patient usual care, current treatment landscapes, long-term clinical outcomes, etc., which can help rare disease manufacturers better understand the impact of the disease on patients and guide future RWE research needs. This is again very pertinent to rare diseases where information can be limited due to the scarcity of patients and challenges of collecting data from these patients. (See “Registries in Rare Disease Research – Approaches to Optimize Success” by Ross in this issue of The Evidence Forum.)
Early engagement in strategic real-world evidence generation planning, in the context of these new opportunities for market access optimization, will be paramount to ensure the right data are available to address peri- and post-approval questions related to product safety, effectiveness, and value. With Germany’s increased focus on long-term, real-world data, manufacturers also need to sharpen their focus to be prepared for these new evidence demands.
For more information, please contact
[email protected], [email protected], [email protected], or
[email protected]