FALL 2019, THE EVIDENCE FORUM, WHITE PAPER
 Marielle Bassel, BA Research Scientist Real-World Evidence Evidera |  Laura Sayegh, MScA Research Associate Real-World Evidence Evidera |  Sofia Fernandes, MSc Associate Director, Project Management Real-World Evidence Evidera |  Delphine Saragoussi, MD, MScPH Senior Research Scientist Real-World Evidence Evidera |
Introduction
The development of new medical treatments follows a well-known pathway from the assessment of safety to the evaluation of therapeutic efficacy, proceeding to pivotal trials to support market authorization decisions.1 Pivotal trials are most commonly designed as traditional randomized clinical trials, designed to maximize the chance of demonstrating safety and efficacy and often include restrictive inclusion and exclusion criteria. While such trials are well suited for that purpose, they can leave evidence gaps, including:
- How the therapy is most impactfully incorporated into clinical practice where there may be other available treatment options
- Real-world safety and effectiveness in the broader patient groups that may receive the treatment upon authorization but for whom limited information is available from the pivotal studies
As a result, regulatory approval of a new treatment is often followed by post-marketing evaluations aimed at addressing a variety of questions, including understanding the real-world setting of care, disease, safety, efficacy, or effectiveness of therapy.1 While there are a number of guidelines and articles that focus on details of the key content of a classic clinical trial protocol, few consider the nuances for protocol design when assessing pre- and post-marketing value in the real-world setting.
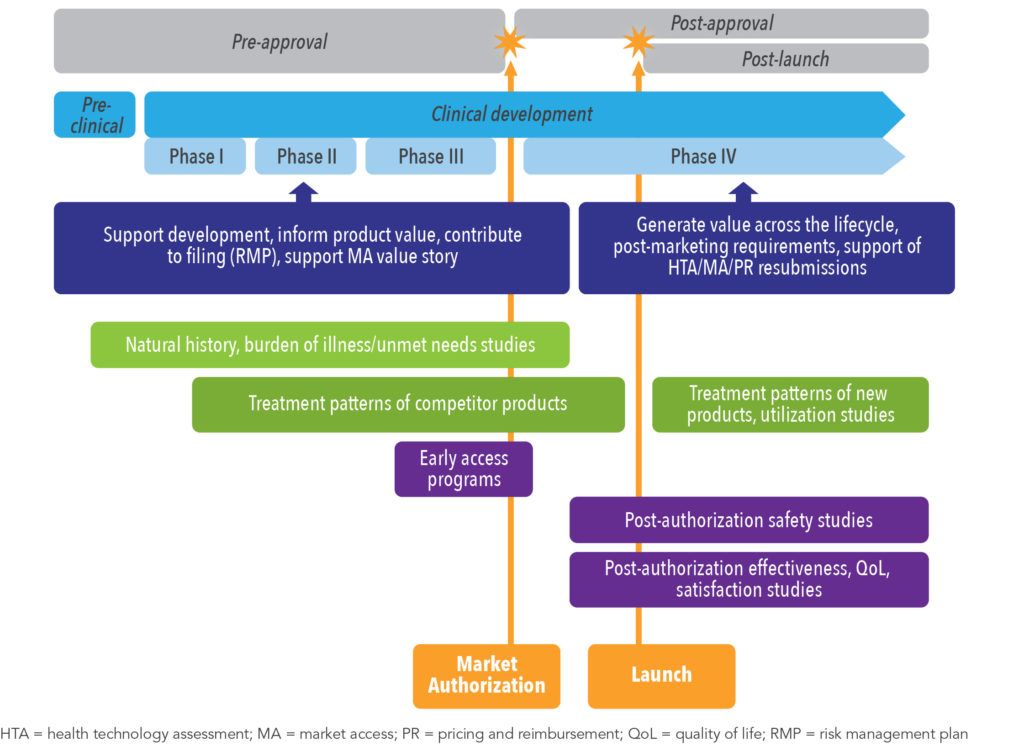
Non-interventional studies, used to generate real-world evidence (RWE), complement and provide additional insight to the data produced through clinical trials.2 Pre-approval designs delineate the natural history and course of disease, standard of care, and contribute to the characterization of burden of illness and unmet needs. Post-approval studies are critical for assessing utilization, treatment patterns, comparative effectiveness and safety, and providing overall value demonstration, as well as informing on important therapeutic findings to help guide treatment decisions and real-world use (See Figure 1).
The creation of a study protocol is pivotal in determining the success of the research effort as it is the fundamental document that drives the study, providing pre-defined, standardized procedural methods to effectively communicate plans for study conduct and implementation to all stakeholders and involved parties. Real-world evidence studies differ from clinical trials in nature as they are devoid of any form of intervention. As patient data are gathered and collected during routine clinical care, specific considerations have to be accounted for when developing non-interventional study protocols.
A good protocol should delineate the research questions and outline the research process, show how the design will help achieve the objectives, demonstrate how the study will be operationalized in practice, highlight its feasibility, and convincingly show the importance of the research.
Stakeholder Involvement in Protocol Development
Similar to clinical trials, an invaluable aspect of non-interventional protocol development is the engagement of the sponsor to identify and involve key stakeholders and critical reviewers. Internal stakeholders ensure the full consistency of the study within the company’s strategy (See Figure 2). External stakeholders might be end users or approvers of the protocol (See Figure 3). Study type, design, and methods need to be adapted to the research questions and objectives but also to the end users and expected applications of the study results. Factors such as study type, design, scope, and research questions may also influence the panel of stakeholders and reviewers based on the study needs and research goals. If, for example, a study includes a rare disease population or an orphan drug, there are benefits in engaging patient community and advocacy groups to gain perspective on the feasibility of the objectives and retention strategies.2 In addition, the conduct of real-world evidence studies requires review by the ethics committees and may be mandated to support regulatory decisions.3
Figure 2. Internal Stakeholder Involvement in Protocol Development 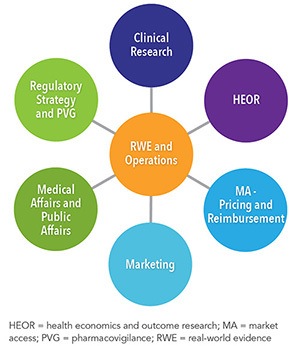 | Figure 3. External Stakeholder Involvement in Protocol Development 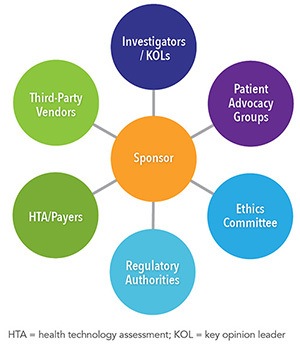 |
Features of an RWE Protocol
Although a real-world study protocol addresses the same principal elements as a clinical trial protocol, there are fundamental differences based on the nature and design of non-interventional studies.2 The content of these protocols can vary widely according to study objectives and design requirements, nevertheless, there is common content to all non-interventional research protocols, which is presented in Table 1.4-9
Key Considerations and Challenges of an RWE Protocol
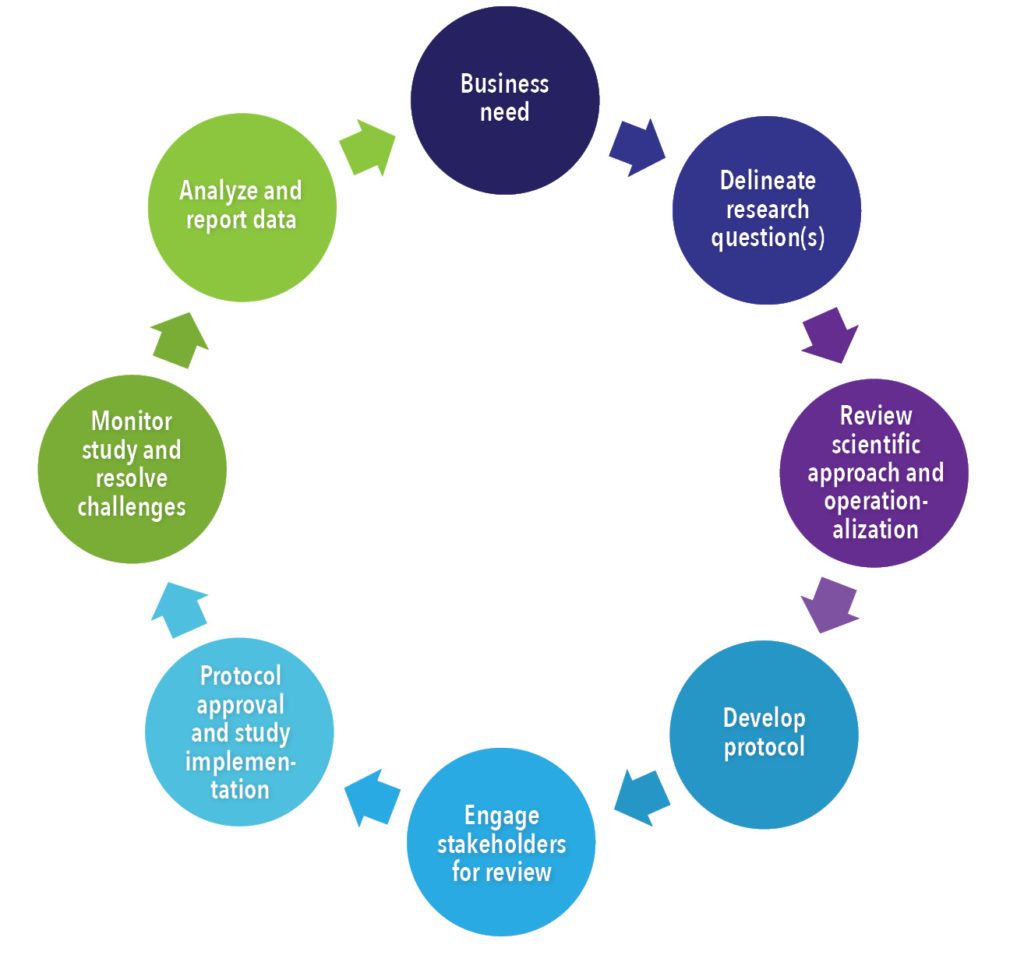
Understanding the underlying rationale behind the sponsor’s needs to conduct the study drives the direction and elements of the protocol development (See Figure 4). To ensure successful design and implementation of the study, there are key factors and challenges to consider. Protocols written by trained individuals with appropriate scientific background, as well as knowledge on safety, product strategy, and market access will help to mitigate and address these issues.
- With the involvement of diverse stakeholders and multiple interests, it is crucial to incorporate feedback, while prioritizing input and maintaining focus on the goal of the study.
- In traditional fixed-design clinical trials, treatment protocols are highly controlled and mandate study visits and adherence to protocol-defined procedures at fixed timepoints.13 Although this approach ensures satisfactory study conduct in a clinical setting, the same might not be permissible in prospective real-world study protocols, especially in some geographical areas where it is paramount to avoid protocol requirements that could impact real-world clinical care and routine clinical practice.
- Addressing real-world outcomes outside of a controlled clinical trial setting requires more flexible data collection. From study design conception, clarity is required in terms of the objectives to permit the selection of the data variables necessary to address the study questions and outcomes. A detailed definition of the variables in the protocol will allow identification of any difficulty upfront and facilitate the creation of the case report form, if any.
- Design and methodological considerations differ depending on the protocol’s intended audience. For example, if the study aim is to provide additional information on post-marketing safety in Europe, then the protocol should adhere to applicable regulatory regulations such as the European Medicines Agency (EMA) Good Pharmacovigilance Practices (GVP) Module VIII,14 and the EMA Post-Authorisation Safety Studies (PASS),15 or abide by European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP).16 These studies may require review and approval from regulatory agencies prior to implementation, and the EMA PASS protocol template or ENCePP protocol checklist17 may need to be consulted during protocol development.
- Adequate time should be taken to coordinate stakeholder input and accurately review the protocol. Discrepancies can lead to amendments or extension of study timelines.18
Key Operational Considerations
As the protocol provides all parties involved in a study a reference document for consultation to assist with study implementation, it is expected that downstream study challenges will have been proactively accounted for during development.
Clinical trial investigators and sites are not always suitable for non-interventional studies, therefore, it is important to perform outreach concurrent to protocol development to identify the most suitable investigators and sites for study participation, while taking into account marketing authorization, healthcare environment and routine clinical care, geographical features, ethics, data protection, notifications to authorities, and reporting requirements. As data sources exist in various formats and systems in the real world, it is critical to determine the best approach for collecting complete and quality data. Thus, collaboration between the protocol writer and operations allows the integration of relevant study details and realistic assumptions into the protocol during its development.
The protocol bridges the gap between the research concept and the study conduct.
Summary

While there are challenges and considerations to drafting all study protocols, those designed for real-world studies have additional layers of complexity as they need to be developed in such a way as not to alter real-world routine clinical care patterns. The protocol, derived from the sponsor’s strategic needs, must guide and enable the collection of robust data and the generation of valid results in the highly variable and dynamic real-world setting, irrespective of the study design and data collection method chosen. Successful study execution is bolstered when the protocol writer is an expert in their field, well versed in the numerous methodological and data collection challenges, and supported by a team of scientific and operational experts. This can also be accomplished when the protocol writer, the sponsor, and critical stakeholders engage in early discussions to clearly define the research questions and delineate the conceptual protocol framework, and then continue to keep an open dialogue throughout the process.
References
- Galson S, Simon G. 2016. Real-World Evidence to Guide the Approval and Use of New Treatments. NAM Perspectives. Discussion Paper, National Academy of Medicine, Washington, DC. doi: 10.31478/201610b. Available at: https://nam.edu/real-world-evidence-to-guide-the-approval-and-use-of-new-treatments/. Accessed September 4, 2019.
- US Food and Drug Administration. Guidance for Industry: Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices. 2017. Available at:
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-real-world-evidence-support-regulatory-decision-making-medical-devices. Accessed July 12, 2019. - Kurz X, Perez-Gutthann S, ENCePP Steering Group. Strengthening Standards, Transparency, and Collaboration to Support Medicine Evaluation: Ten Years of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP). Pharmacoepidemiol Drug Saf. 2018 Mar;27(3):245-252. doi: 10.1002/pds.4381. Epub 2018 Jan 11.
- Al-Jundi A, Sakka S. Protocol Writing in Clinical Research. J Clin Diagn Res. 2016 Nov;10(11):ZE10-ZE13. doi: 10.7860/JCDR/2016/21426.8865. Epub 2016 Nov 1.
- Biostatistics Group, UCLH/UCL/RFH Biomedical Research Unit. Guidelines for Completing a Research Protocol for Observational Studies 2010. Available at: https://icahn.mssm.edu/files/ISMMS/Assets/Research/IHCDS/Guidelines%20for%20Completing%20a%20research%20protocol%20for%20observational%20studies.pdf. Accessed July 15, 2019.
- US Food and Drug Administration. Guidance for Industry: Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment. 2005. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/good-pharmacovigilance-practices-and-pharmacoepidemiologic-assessment. Accessed August 16, 2019.
- Government of Canada. Elements of Real World Data/Evidence Quality throughout the Prescription Drug Product Life Cycle. 2019. Available at: https://www.canada.ca/en/services/health/publications/drugs-health-products/real-world-data-evidence-drug-lifecycle-report.html. Accessed July 15, 2019.
- Hoffmann W, Latza U, Baumeister SE, et al. Guidelines and Recommendations for Ensuring Good Epidemiological Practice (GEP): A Guideline Developed by the German Society for Epidemiology. Eur J Epidemiol. 2019 Mar;34(3):301-317. doi: 10.1007/s10654-019-00500-x. Epub 2019 Mar 4.
- MUHC Research Ethics Board (Neurosciences & Psychiatry). Protocol Drafting Guideline from MUHC RI SOP SOP-CR003-EN04_Appendix 1. 2016. Available at: https://www.mcgill.ca/neuro/files/neuro/protocoledrafingguide_2mar2016.pdf. Accessed July 15, 2019.
- International Council for Harmonisation of Technical Requirement for Pharmaceuticals for Human Use (ICH). Guideline for Good Clinical Practice. 2016. Available at: https://www.ich.org/home.html. Accessed July 12, 2019.
- International Society for Pharmacoepidemiology. Guidelines for Good Pharmacoepidemiology Practices (GPP). 2015. Available at: https://www.pharmacoepi.org/resources/policies/guidelines-08027/. Accessed July 23, 2019.
- International Council for Harmonisation of Technical Requirement for Pharmaceuticals for Human Use. General Considerations for Clinical Studies (Draft). 2019. https://www.ich.org/home.html. Accessed July 30, 2019.
- US Food and Drug Administration. Framework for FDA’s Real-World Evidence Program. 2018. Available at: https://www.fda.gov/media/120060/download. Accessed July 12, 2019.
- European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP). EMA/395730/2012 Rev 2*. 2016. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-viii-addendum-i-requirements-recommendations_en.pdf. Accessed August 16, 2019.
- European Medicines Agency. Guidance for the Format and Content of the Protocol of Non-Interventional Post-Authorisation Safety Studies (PASS). EMA/623947/2012. 2012. Available at: https://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/10/WC500133174.pdf. Accessed July 12, 2019.
- European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP). Guide on Methodological Standards in Pharmacoepidemiology (Revision 5). 2010. Available at: http://www.encepp.eu/standards_and_guidances/. Accessed July 16, 2019.
- European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP). ENCePP Checklist for Study Protocols (Revision 4), EMA/540136/2009. 2009. Available at: http://www.encepp.eu/standards_and_guidances/checkListProtocols.shtml. Accessed July 16, 2019.
- Kendle K. Clinical Study Protocols: How to Write to Solve Problems Now and Avoid Big Ones in the Future. Medical Writing: The Backbone of Clinical Development. 2017:25-27. Available at: https://www.certara.com/wp-content/uploads/Resources/Articles/AR_ClinicalStudyProtocols.pdf. Accessed September 4, 2019.
For more information, please contact us.