FALL 2019, THE EVIDENCE FORUM, WHITE PAPER
 |
| Ann Menzie, MS Senior Director Evidence Synthesis, Modeling & Communication Evidera |
Medical devices are part of everyday life and essential throughout all areas of healthcare, including prevention, diagnosis, and treatment. They include any device intended for medical purposes, such as instruments, implants, machines, materials, software, etc., and range from tongue depressors and blood pressure cuffs, to cardiac stents and joint replacements, to surgical robots and software. Innovation of medical devices is often an iterative development process based on recognized need rather than transformational improvement to address a unique, unmet clinical need. As a result of the iterative nature of medical device development, little, if any, clinical evidence showing improvement in outcomes is available to support the product launch. Hospitals, ambulatory surgical centers, and physician offices are the primary buyers of medical devices and frequently view them as commodities. Most hospitals have implemented cross-functional value analysis teams to evaluate the clinical and economic impact of adopting new technologies, including medical devices. The result is a crisis where medical device manufacturers are facing extreme pricing pressure on both new and existing products and are being asked by hospitals to provide evidence to support product claims and value propositions – evidence the manufacturers often do not have. In this environment, there is a significant need for manufacturers to invest in evidence generation to change the discussion with hospitals from price to value.
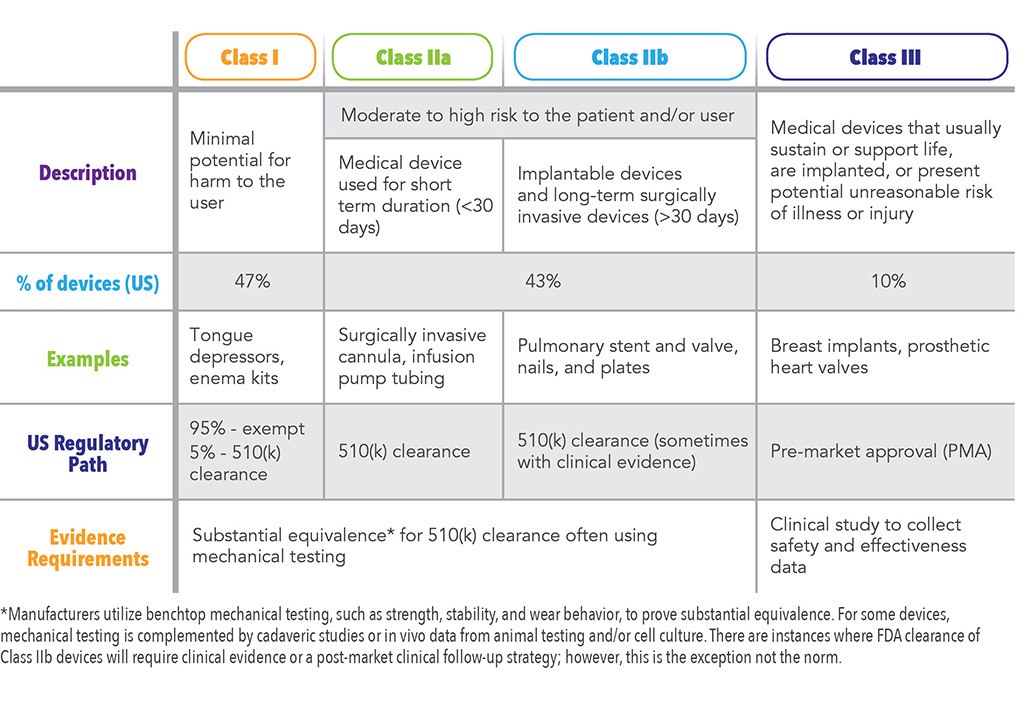
Unlike the pharmaceutical industry, medical devices often do not require clinical evidence for US Food and Drug Administration (FDA) approval prior to launch. Medical devices are classified by global regulatory authorities using a risk-based classification system: Class I (lowest risk to patients), Class II, and Class III (highest risk to patients). This classification system is used in most global markets and includes four categories (Class I, Class IIa, Class IIb, and Class III). In the US, only 10% of medical devices are classified as Class III and require clinical safety and efficacy data for FDA approval.1 A summary of these categories is shown in Table 1.
While hospitals, physicians, and payers request clinical and economic data to inform evidence-based decisions regarding new medical devices, manufactures do not commonly invest in these studies prior to product launch because they have not historically been a requirement for regulatory approval. It is important to note, however, that the regulatory processes are evolving, particularly in the European Union (EU), and new evidence requirements for medical devices are being implemented beginning May 2020.
Manufacturers of medical devices must, therefore, evaluate the impact of investing in post-market clinical trials. While there is often a substantial upside to collecting clinical data on new products, particularly to support claims targeting physicians and hospitals, these studies are costly and time consuming to design and execute. New product innovation is often iterative with new products or line extensions occurring roughly every two to three years. It is not uncommon for the pace of new product launches to exceed the timeline for the clinical trial. Manufacturers must evaluate if the clinical study is worth the investment if the study timeline results in publications reporting outcomes on a previous generation technology.
Medical device companies are leveraging evidence in many ways to maximize their businesses, including driving innovation, supporting evidence-based pricing strategies, and addressing future regulatory evidence requirements. Evidence generation strategies often include a combination of study designs and geographic locations.
Real-world data (RWD) and real-world evidence (RWE) have become integral parts of global evidence generation strategies. RWD refers to data derived from a wide range of sources relating to patient health and healthcare resource utilization,3 including electronic health records, administrative claims and billing data, patient registries, and mobile devices. RWE is derived from the analysis of RWD and includes clinical evidence reporting usage of a medical device as well as associated benefits or risks.3 RWE is used to complement post-launch clinical trial data to create a robust evidence base showing safety and efficacy in a defined patient population as well as outcomes in the general population.
Using RWE to Drive Medical Device Innovation
RWE provides a means of revealing markets that are ripe for disruption based on unmet clinical needs. For example, administrative claims databases house de‑identified patient data, including medical diagnoses and procedures, prescribed medications, and healthcare costs. Patient cohorts can be identified using procedure or diagnosis codes and their healthcare resource utilization can be tracked longitudinally. Procedures with high rates of complications and revisions using existing technologies are prime targets for innovation. Additionally, patient subgroups that are at higher risk for adverse events can be identified and targeted for new therapies. RWE analyses are most impactful when coupled with literature searches and clinician feedback to complement the identification of opportunities for innovation that improve patient care.
In addition to using RWE to reveal unmet clinical needs, medical device companies leverage RWD to assess the economic burden of current treatments and identify focus areas for innovation. Providers have a financial interest in reducing the overall cost of care to patients and the healthcare system by adopting technologies that reduce the total cost of care by addressing key economic drivers. These economic drivers may include reducing costly postoperative complications and revisions, time in the Intensive Care Unit (ICU), hospital length of stay, and allowing home discharge status after a surgical procedure compared to more costly alternatives, such as skilled nursing facilities.
Leveraging RWE to Inform Evidence-Based Pricing Strategies
RWE also serves as a key resource for medical device manufacturers for evidence-based pricing of new products. For example, if a medical device is designed to reduce post-operative complications, hospital length of stay, time in the ICU, and/or operating room time, then these opportunities for hospital cost savings should be captured in evidence-based pricing strategies. Manufacturers may leverage hospital administrative databases to assess the cost of the surgical procedure, the length of stay, and cost of revision procedures, and provide insights related to the cost of post-operative care. Understanding where the new product innovation will deliver value to the healthcare system will provide critical inputs into a pricing strategy that succeeds in delivering value to customers while not leaving money on the table for device manufacturers.
Use of RWE to Support Regulatory Requirements for Evidence
Within the US, the FDA has issued guidelines for using RWE from electronic health records, registries, and administrative claims data to support regulatory decision making.3 This document provides guidance for industry regarding the use of RWE to inform or augment data used to develop the benefit-risk profile provided to the FDA.3 These data may provide new insights into the usage patterns, performance, and clinical outcomes associated with medical devices and may be used by manufacturers to show compliance with regulatory requirements.3 Additionally, RWE will be used in the future to help monitor post-market performance of medical devices. The FDA has developed plans to implement the National Evaluation System for health Technology (NEST), which will utilize RWE to identify safety issues and risks of medical devices used in clinical care.3 The implementation of NEST is an important step in monitoring medical device safety data and facilitating rapid identification of safety signals that may trigger the need for a device recall. There is a global aim to collect and monitor safety data to protect patients from devices with early failures and other adverse events.
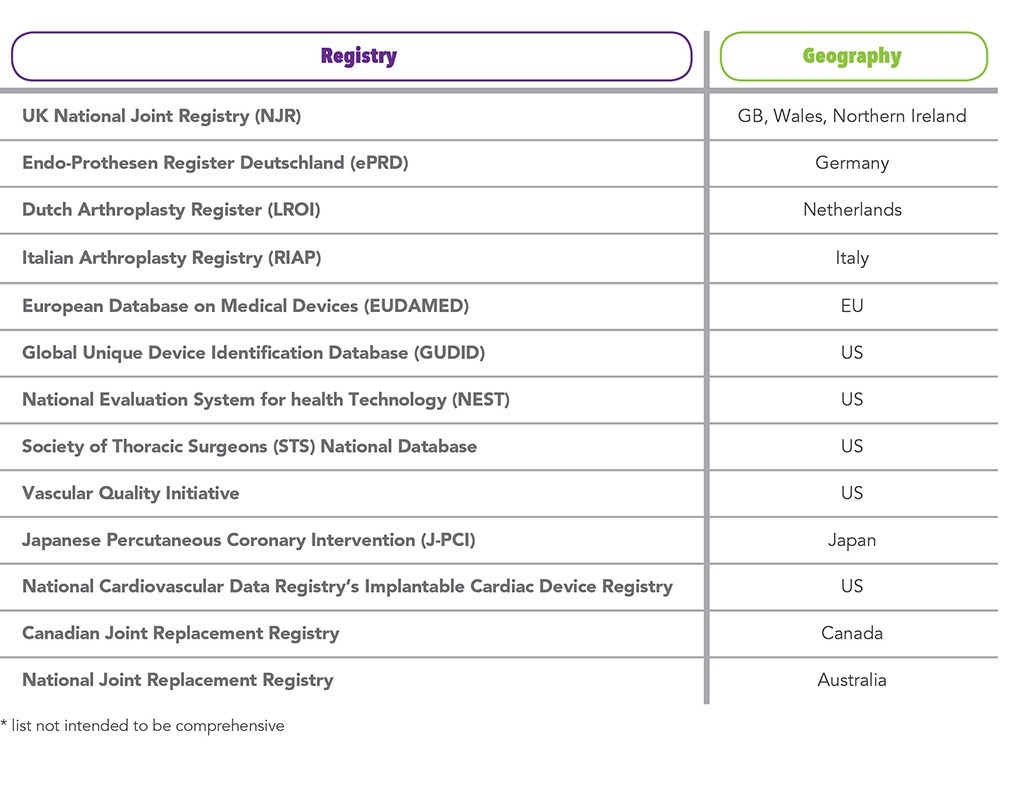
Outside of the US, there is a strong effort to reclassify many surgical implants, such as surgical mesh and spinal implants, from Class II to Class III. In doing so, the evidentiary requirements for regulatory approval will increase substantially. The Medical Device Regulation (MDR) in the EU is a driving force behind this change, and countries such as Australia are considering following suit. RWE will be an important tool in this data collection effort to complement clinical studies to achieve marketing authorization in the EU. MDR will also require robust post-market surveillance (PMS) or post-market clinical follow-up (PMCF) to collect data on safety and performance of the device throughout its entire lifetime.4 Administrative databases, registries, surveys, and electronic health records will be valuable resources for the PMCF effort that will be required by the new MDR initiative going into effect in May 2020. Table 2 shows examples of surveys and registries from across the globe being utilized to track safety and performance of medical devices.
Considerations for Future Use of RWE
In the future, devices will capture their own data. Implants will have chips that evaluate rate of healing; technologies will monitor for signs of infection; and, wearables will collect ongoing data on gait, movement, and health status. Mobile health apps will collect data on a patient’s compliance with hospital discharge instructions and may create a communication channel between the patient and their healthcare team. Medical devices will be tracked throughout their lifetime through unique device identification (UDI), allowing for an even greater degree of device performance and safety analysis. The massive amount of data generated by registries, health apps, and smart devices will create opportunities for companies from other industries to emerge in the medical device space to collect and analyze the data. However, researchers will face challenges to ensure the data analyses are of sound, scientific design and are disseminated in a meaningful way.
In conclusion, this is an exciting time for the medical device industry to harness the power of RWE to drive innovation and support business needs. These data will also be leveraged by payers, hospitals, physicians, and patients to make evidence-based decisions regarding the use of new technology and its value to the healthcare system. With the evolution of evidence requirements for medical devices indicating a greater need for clinical and real-world evidence for both approval and market access, manufacturers are paying more attention to their evidence generation plans for devices and diagnostics, and the benefit could be substantial.
Special thanks to Malinda O’Donnell, MSc, Executive Director and General Manager, Market Access Communications; and Leigh Ann White, Executive Director, Client Services, Evidence Synthesis, Modeling & Communication, for their expert review of this article.
References
- U.S. Food and Drug Administration. Learn if a Medical Device Has Been Cleared by FDA for Marketing. December 20, 2017. Available at: https://www.fda.gov/medical-devices/consumers-medical-devices/learn-if-medical-device-has-been-cleared-fda-marketing. Accessed July 19, 2019.
- European Commission. Medical Devices: Guidance Document – Classification of Medical Devices. MEDDEV 2. 4/1 Rev. 9, June 2010. Available at: http://ec.europa.eu/DocsRoom/documents/10337/attachments/1/translations. Accessed July 19, 2019.
- U.S. Food and Drug Administration. Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices – Guidance for Industry and Food and Drug Administration Staff. August 2017. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-real-world-evidence-support-regulatory-decision-making-medical-devices. Accessed July 19, 2019.
- Melvin T, Torre M. New Medical Device Regulations: The Regulator’s View. EFORT Open Rev. 2019 Jun 3;4(6):351-356. doi: 10.1302/2058-5241.4.180061. eCollection 2019 Jun.
For more information, please contact us.