FALL 2019, THE EVIDENCE FORUM, WHITE PAPER
 |
| Kevin Marsh, PhD Executive Director, Commercial Strategy & New Product Development Patient-Centered Research Evidera |
Introduction
Healthcare decision making involves value judgements, such as whether the benefits of a treatment outweighs its risks, whether the benefits associated with a therapy are worth its cost, or which patient groups’ outcomes should be prioritized for funding. Decision makers are increasingly interested in using quantitative preference data on how stakeholders make such trade-offs to support their decisions. For instance, the US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) encourages manufacturers to submit patient preference information (PPI) to support its benefit-risk assessment.1
Health technology assessment (HTA) often also involves the use of quantitative preference data, with general population preferences being the basis for the calculation of the tariffs used to estimate utility inputs for the cost-effectiveness analysis.2 That is, a societal perspective is often adopted. While patient input is sought, often in the form of qualitative insights on the burden of the disease, submissions from patient advocacy groups, or patient representatives being members of decision-making committees,3 there has traditionally been little or no role in HTA for quantitative PPI.
Recently, however, this has started to change. Across Europe, HTA agencies are consulting on the use of PPI, setting precedents by considering it in their decision making, and providing advice on its use. In some instances, the role of PPI has been formalized in methods guidance. This article provides a snapshot on the use of PPI in Europe and reflects on how its use may change in the future.
The Use of PPI by HTA in Europe
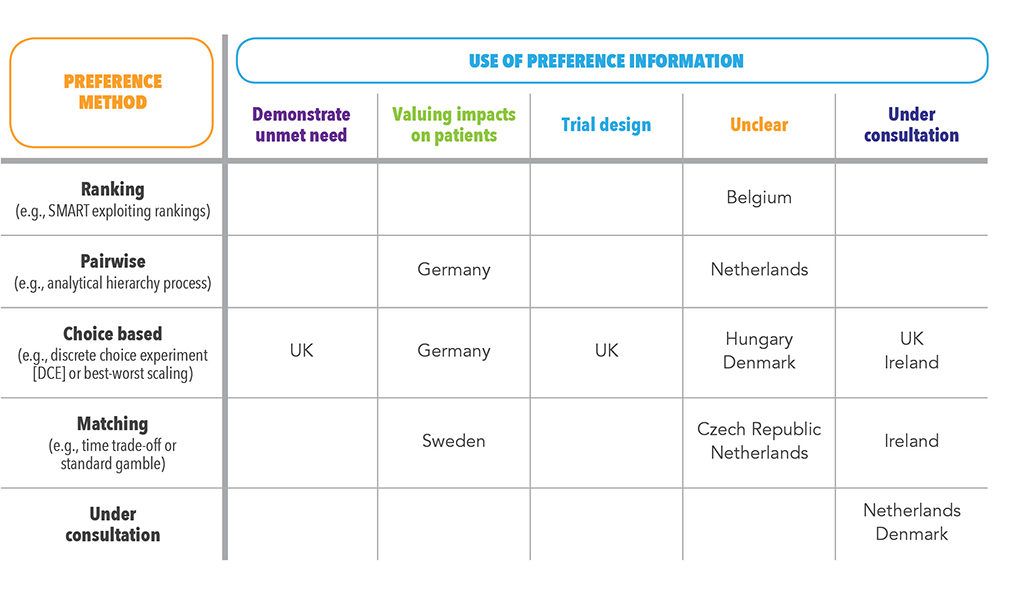
An ongoing ISPOR working group has mapped the use of PPI by HTA agencies in Europe.4 The mapping involved a literature review; a review of agency websites; a survey of agency staff; and, a consultation with local experts. The results of this review, supplemented by more recent examples of the use of PPI by HTA agencies in Europe, are summarized in Table 1.
The table illustrates how agencies in key markets – in particular Germany, Sweden, and the UK – are leading the use of PPI. In Germany and Sweden, the goal of PPI use has been to base economic evaluation on a more accurate estimate of the value of impacts on patients than would be generated by the QALY. In Germany, the Institute for Quality and Efficiency in Healthcare (IQWiG) has recommended in its method guide that PPI be used to estimate the aggregate benefit in an economic evaluation.5 In Sweden, it is recommended that PPI be used where the QALY is thought to be inappropriate, such as when valuing changes in short-term pain.
In the UK, PPI has been used by the National Institute for Health and Care Excellence (NICE) in two other ways – in the unmet need section of the submission, demonstrating the value that patients place on finding alternative modes of administration6; and to inform the selection of endpoints that are included in a trial. The latter use was the subject of a recent scientific advice offered by NICE on the design of a trial for a COPD treatment. In their press release advertising that they’d provided the scientific advice, NICE stated that it was their aim “to encourage more companies to seek its advice on the development of these studies … so they can be used in the clinical development programs.7”
These examples represent the better documented use of PPI in HTA. But the use of PPI may be broader than examples suggest. The ISPOR review reported expert testimony that PPI has been used in reimbursement submissions in Belgium, the Czech Republic, Denmark, Hungary, and the Netherlands.4 The precise use of PPI wasn’t clear from this data.
Ongoing consultations also point to a broadening use of PPI in the future. Pilots and consultation on the use of PPI by HTA agencies were identified by Denmark, Ireland, the Netherlands, and the UK. Exploration of the use of PPI in HTA is also being supported by the Innovative Medicines Initiative’s Patient Preferences in Benefit-Risk Assessments during the Drug Life Cycle (PREFER) project. Its objective is to generate recommendations on when and how to collect and use PPI to support decision making by industry, regulatory authorities, and HTA bodies.8 PREFER has established a formal structure to incorporate input from reimbursement agencies into its activities, with representation from agencies from Austria, Belgium, and Germany on its Stakeholder Advisory Group.
Challenges to Incorporating PPI into HTA
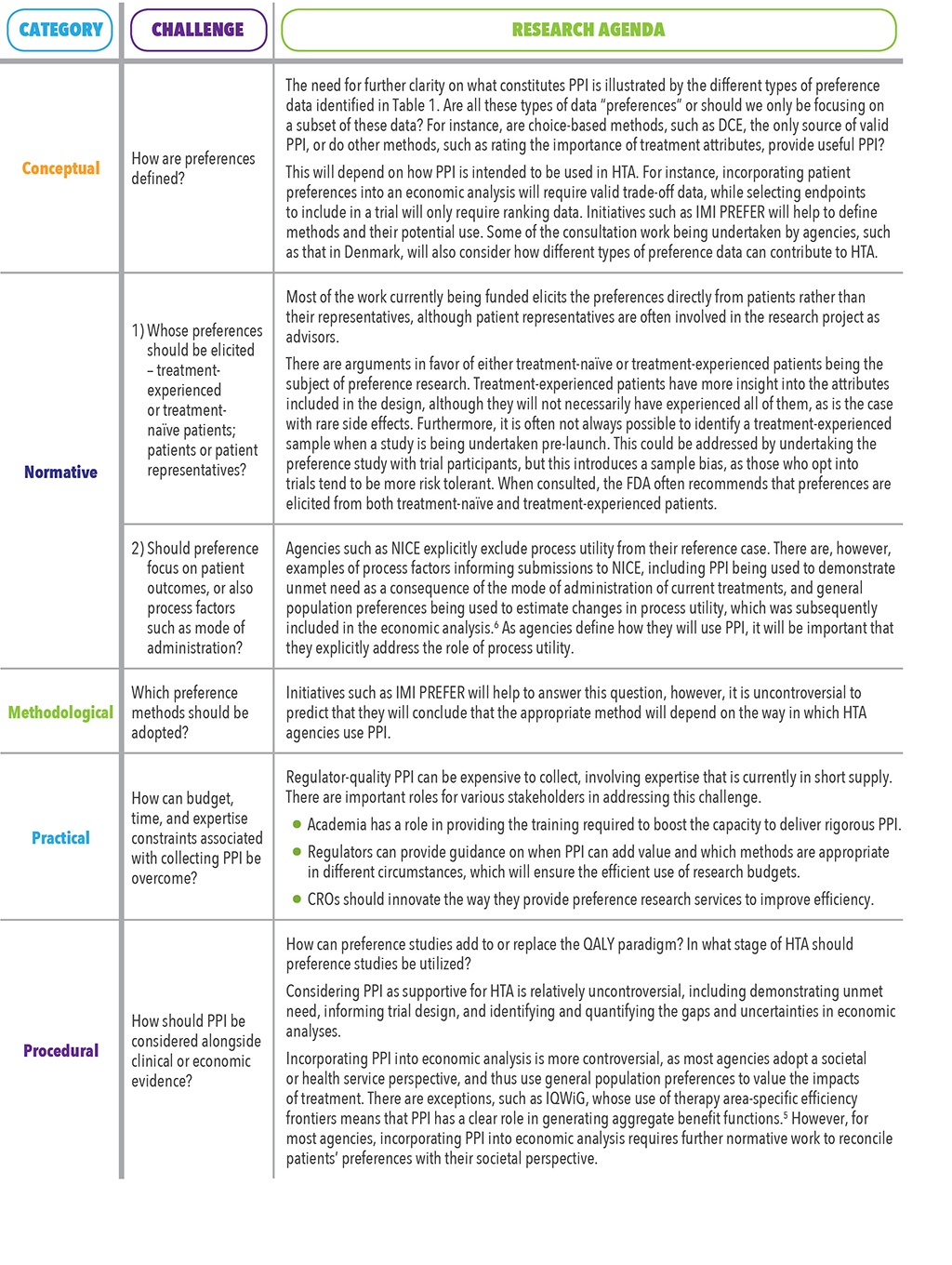
Despite the increased interest of HTA agencies, the use of PPI in reimbursement decisions raises a number of issues. Five categories of challenges were identified by a recent review by Huls et al.9 These are summarized in Table 2 with reflections on the implications for the use of PPI in HTA.
Conclusion
PPI is increasingly used to support regulatory decisions, and sponsors and HTA agencies are actively exploring how this data can also support reimbursement decisions. This latter effort is still in its exploratory phase. A small number of HTA agencies have specified the use of PPI in their methods guidance, but most agency use of PPI is less systematic, either being in the form of novel examples of the use of PPI or at a pilot stage. These case studies and pilots point to the likely increase in the use of PPI for HTA. Where the existing methods fail to capture the value of technologies to patients – e.g., improvements in the mode of administration or health impacts that are not easily captured in the QALY, such as acute pain – PPI has a role to play in HTA. There are issues to be addressed, however, before this role becomes clear. Ongoing initiatives will help provide insight into some of these questions and concerns. In the meantime, sponsors considering the use of PPI are advised to consult agencies on a case-by-case basis to consider its acceptability and likely impact.
References
- US Food and Drug Administration. Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. August 24, 2016. Available at: https://www.fda.gov/media/92593/download. Accessed September 5, 2019.
- National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9]. April 2013. Available at: https://www.nice.org.uk/process/pmg9/chapter/foreword. Accessed September 5, 2019.
- Health Technology Assessment International. Good Practice Examples of Patient and Public Involvement in Health Technology Assessment. February 2015. Available at: https://htai.org/wp-content/uploads/2018/02/Good_Practice_Examples_Feb_2015.pdf. Accessed September 5, 2019.
- ISPOR Stated Preference Special Interest Group. Workshop: Preference Research in HTA Agencies Europe – A Review of Its Use in Approval, Reimbursement and Pricing. Presented at ISPOR Europe 2018, Barcelona, Spain, November 12, 2018.
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). General Methods, Version 5.0 / 10.07.2017: Köln; 2017 Available at: https://www.iqwig.de/download/Allgemeine-Methoden_Version-5-0.pdf. Accessed September 4, 2019.
- National Institute for Health and Care Excellence (NICE). Technology Appraisal Guidance [TA466]. Baricitinib for Moderate to Severe Rheumatoid Arthritis. 09 August 2017. Available at: https://www.nice.org.uk/guidance/ta466. Accessed September 4, 2019.
- National Institute for Health and Care Excellence. NICE Provides First Scientific Advice on Patient Preference Study Design. Available at: https://www.nice.org.uk/news/article/nice-provides-first-scientific-advice-on-patient-preference-study-design. Accessed August 30, 2019.
- PREFER Patient Preferences. Available at: https://www.imi-prefer.eu/. Accessed August 30, 2019.
- Huls SPI, Whichello CL, van Exel J, Uyl-de Groot CA, de Bekker-Grob EW. What is Next for Patient Preferences in Health Technology Assessment? A Systematic Review of the Challenges. Value Health. In Press. Available online 3 August 2019. https://doi.org/10.1016/j.jval.2019.04.1930.
For more information, please contact us.