SPRING 2020, THE EVIDENCE FORUM, WHITE PAPER
 |
| Donald Smith, PhD Scientific Director Market Access Communications Evidera |
On December 23, 2019, the Academy of Managed Care Pharmacy (AMCP) released Version 4.1 of the AMCP Format for Formulary Submissions.1 This new version of the AMCP Format describes three types of dossiers that manufacturers may choose to develop: Unapproved Product Dossiers, Approved Product Dossiers, and Unapproved Use Dossiers.2
Unapproved Product Dossiers provide information about a product that is not currently approved by the United States (US) Food and Drug Administration (FDA).2 Manufacturers may use these dossiers to provide information about a product to healthcare decision makers (HCDMs) prior to FDA approval.2
Approved Product Dossiers present information about a product that has been approved by the FDA.2 Manufacturers may use these dossiers to reactively provide information about a product to HCDMs in response to an unsolicited request after FDA approval.2
Unapproved Use Dossiers contain information about a product that is currently approved by the FDA.2 However, this information focuses on a currently unapproved indication of the approved product.2 Manufacturers may use these dossiers to inform HCDMs about an unapproved use of an approved product prior to FDA approval of the unapproved use.2
While developing Version 4.1 of the AMCP Format, the AMCP Format Executive Committee decided to focus their updated recommendations on Unapproved Product Dossiers and Unapproved Use Dossiers, and made minimal changes to the section on Approved Product Dossiers.2 Therefore, a piece of good news for manufacturers is that converting an existing Post-Approval Dossier that follows Version 4.0 of the AMCP Format into an Approved Product Dossier that follows Version 4.1 of the AMCP Format is a straightforward process. For these reasons, this article will focus on Unapproved Product Dossiers and Unapproved Use Dossiers.
Why Develop an Unapproved Product Dossier or Unapproved Use Dossier?
Even though manufacturers are not required to develop Unapproved Product Dossiers or Unapproved Use Dossiers,2 they can be very useful tools. Version 4.1 of the AMCP Format specifically states that “HCDMs need and are interested in receiving information from manufacturers about unapproved products and about unapproved uses of approved products for which FDA approval is being sought.”2 However, it is important to note that manufacturers may also benefit from developing dossiers that describe an unapproved product or an unapproved use of an approved product. For example, developing an Unapproved Product Dossier or Unapproved Use Dossier may be helpful if:
- The manufacturer is entering a new field and is not familiar with the disease and/or its key treatments
- The manufacturer wants to start work on an AMCP dossier for internal needs, but feels that it is too early to start work on an Approved Product Dossier
- The manufacturer wants to understand how the available literature supports their early, and often aspirational, value story and what evidence gaps exist
- The product will have an orphan or fast-track designation
- The product is not being launched outside of the US, and therefore there is no global value dossier, making the AMCP dossier the primary source for information related to the clinical and economic value of the product
- The clinical development plan for a product includes multiple indications or disease populations
- The manufacturer wants to share information with HCDMs prior to FDA approval of the unapproved product or the unapproved use
Finally, unlike Approved Product Dossiers, which can only be provided in response to an unsolicited request (i.e., reactively), Unapproved Product Dossiers and Unapproved Use Dossiers may be provided either proactively or reactively, at the discretion of the manufacturer.2 However, many HCDMs want the information contained in these dossiers as early as possible so that they can more effectively plan for future drug approvals. Therefore, it is expected that many HCDMs will request Unapproved Product Dossiers and Unapproved Use Dossiers, and that most of these dossiers will be provided in response to an unsolicited request rather than in a proactive manner.
An Important Question
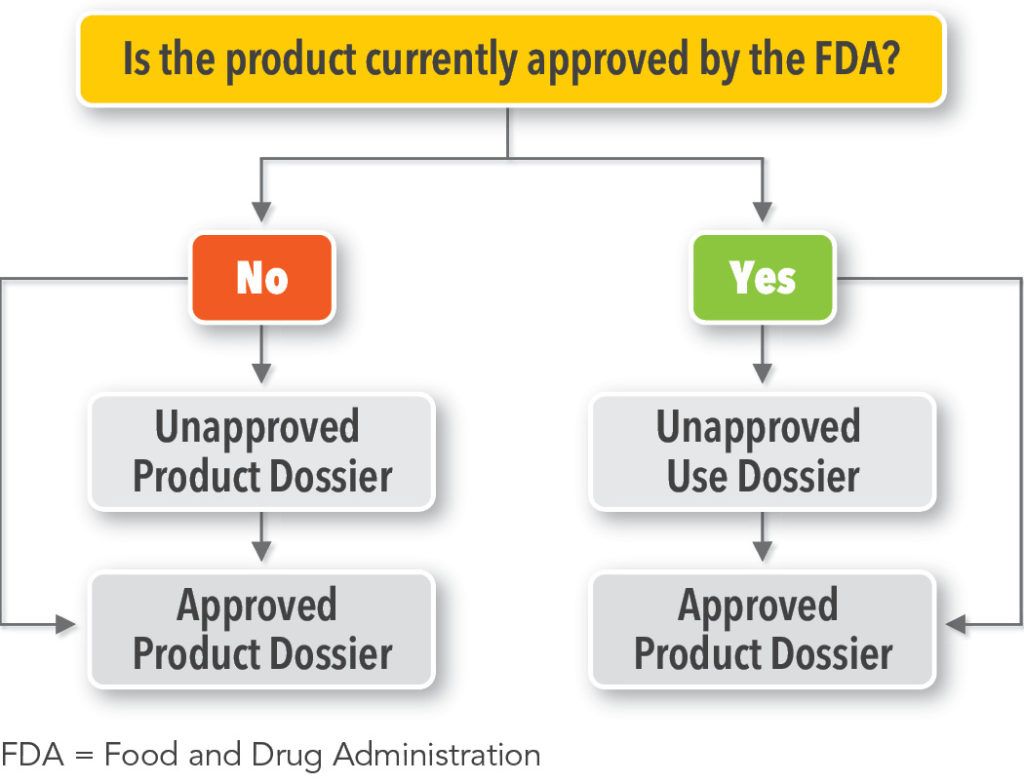
When deciding which new dossier type(s) to develop, it is important to ask whether the product of interest is currently approved by the FDA.
- If the product is not currently approved by the FDA, the manufacturer may develop an Unapproved Product Dossier and/or an Approved Product Dossier (See Figure 1). The manufacturer is not obligated to develop an Unapproved Product Dossier prior to developing an Approved Product Dossier.2
- If the product is currently approved by the FDA, the manufacturer may develop an Unapproved Use Dossier and/or an Approved Product Dossier (See Figure 1). There is no requirement for a manufacturer to develop both an Unapproved Use Dossier and an Approved Product Dossier.2
Unapproved Product Dossiers and Unapproved Use Dossiers
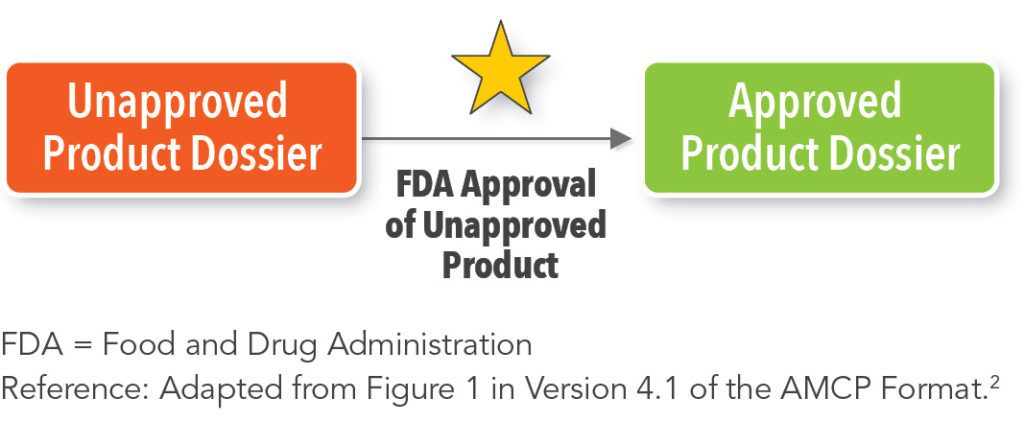
As shown in Figure 2, an Unapproved Product Dossier should be converted into an Approved Product Dossier when the unapproved product receives approval from the FDA.2 An Unapproved Product Dossier and an Approved Product Dossier for the same product never exist simultaneously; a product is either approved by the FDA or it is not.2
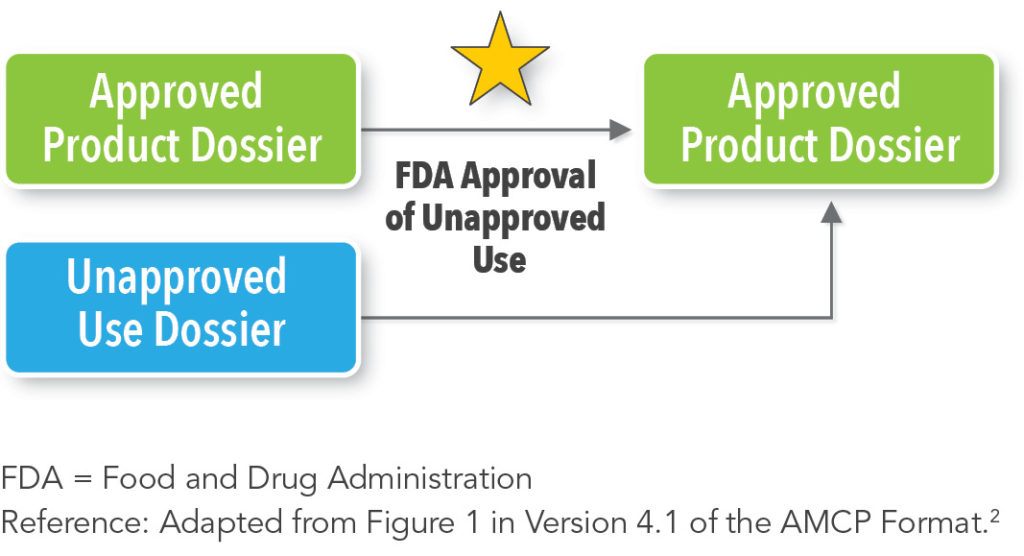
In contrast, an Unapproved Use Dossier and an Approved Product Dossier may exist simultaneously; this depends upon the product and the goals of the manufacturer (See Figure 3).2 However, the information in the Unapproved Use Dossier should be incorporated into the existing Approved Product Dossier after FDA approval.2 Alternatively, an Unapproved Use Dossier could become its own Approved Product Dossier,2 and the manufacturer could ultimately have multiple Approved Product Dossiers for a single product, a practice that has existed for years. Interestingly, some of the information that appears in an Unapproved Use Dossier may originate in an Approved Product Dossier since Approved Product Dossiers may contain information about potential off-label uses and potential new indications under investigation.2
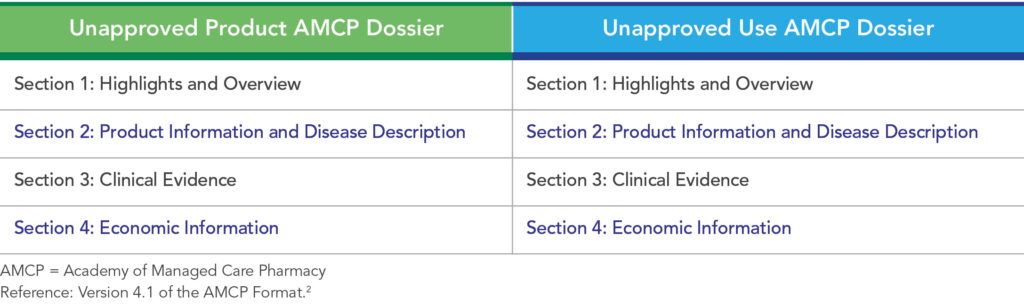
Per Version 4.1 of the AMCP Format, Unapproved Product Dossiers and Unapproved Use Dossiers should contain the same four main sections (See Table 1).
Section 1: Highlights and Overview
Unapproved Product Dossiers and Unapproved Use Dossiers begin with a Highlights and Overview section that consists primarily of a table and outlines some of the important general information about a product, such as its name, expected approval date, clinical trials, and expected indication.2 There is no Executive Summary in an Unapproved Product Dossier because:
- “Manufacturers may not make claims about an unapproved product”2
- “Manufacturers may not make claims about an unapproved use of an approved product”2
- “No characterizations or conclusions should be made regarding the safety or effectiveness of the unapproved product or the unapproved use”3
Instead, the manufacturer should focus on providing information that is factual, objective, and unbiased.2,3 Version 4.1 of the AMCP Format contains a table that provides guidance on the type of information that should be included in Section 1 of an Unapproved Product Dossier and Unapproved Use Dossier.2 Some examples include the New Drug Application (NDA) or Biologics License Application (BLA) submission date, the Prescription Drug User Fee Act (PDUFA) or FDA approval date, information on Phase II and Phase III trials, the prevalence and incidence of the disease in the US, and information on product pricing.2 Of note, the manufacturer is expected to include the current list price of the approved product in an Unapproved Use Dossier.2
Section 2: Product Information and Disease Description
In Section 2.1 (Product Description), the manufacturer should include general information about the unapproved product, such as its mechanism of action, the patient population being examined, projections related to patient utilization, and patient support programs.2 Importantly, as is typically done for Approved Product Dossiers, the current version of the FDA approved prescribing information should be attached to an Unapproved Use Dossier.2
In Unapproved Product Dossiers, the manufacturer must include a clear statement that “the unapproved product is not FDA approved, and that the safety or effectiveness of the unapproved product has not been established.”2,3 Similarly, an Unapproved Use Dossier must include a clear statement that “the unapproved use of an approved product is not FDA approved, and that the safety or effectiveness of the unapproved use has not been established.”2,3
In Section 2.2 (Disease Description), the goal is to describe the disease, including information on epidemiology, clinical presentation, and disease burden. At a webinar conducted by AMCP on January 23, 2020, a question was raised as to why including information on unmet need, treatment guidelines, and competitors/comparators in Unapproved Product Dossiers and Unapproved Use Dossiers is not specifically mentioned in Version 4.1 of the AMCP Format.4 The answer given at the webinar centered around a reminder that no characterizations, conclusions, or claims may be made about a product that is not approved by the FDA or about an unapproved use of an approved product.4 However, it was also mentioned that Unapproved Product Dossiers and Unapproved Use Dossiers may discuss the current state of the field in general.4 For example, an Unapproved Product Dossier can discuss the current unmet need in a field, but it cannot state or imply that the unapproved product fulfills that unmet need.4 Similarly, information from treatment guidelines and data on potential competitors/comparators can be included in an Unapproved Use Dossier.4 However, the information presented must be factual and unbiased, and no characterizations, conclusions, or claims about the unapproved use of the approved product may be made or implied.4
Section 3: Clinical Evidence
In Section 3 of Unapproved Product Dossiers and Unapproved Use Dossiers, the manufacturer can discuss the clinical trial program that supports the unapproved product or unapproved use of interest. Information that may be included in this section includes publicly available information describing the design, patient population, and results of the studies.2 Data on file that is not publicly available can be included at the discretion of the manufacturer.2
Manufacturers should focus on making a factual presentation that does not include claims, characterizations, or conclusions.2 Therefore, a “Conclusions” section should not be included for the study summaries. However, whether the studies should be presented as text-based study summaries, evidence tables, or both is up to the manufacturer.2
Section 4: Economic Information
Unapproved Product Dossiers
The last section of an Unapproved Product Dossier, Economic Information, has generated a fair amount of discussion. This is mostly because it is uncommon for manufacturers to provide information about the potential price of a product prior to approval by the FDA.2 The intent of this section is to provide HCDMs with information on pricing before FDA approval so that they can plan for future reimbursement decisions in a more informed manner.2 While the AMCP Format Executive Committee “strongly recommends that manufacturers provide as much product pricing information as possible,” the manufacturer is under no obligation to provide this information.2
If a manufacturer chooses to provide information on the potential pricing of an unapproved product prior to FDA approval, the price may be provided as a range.2 In fact, Version 4.1 of the AMCP Format helpfully provides price ranges that can be used for this purpose.2 However, even if an informed price range is provided by the manufacturer, it may not be possible to develop economic models prior to FDA approval.2 This is because it is likely that economic models will include outcomes and/or assumptions related to the effectiveness and safety of the unapproved product in the target population.2
Unapproved Use Dossiers
The section on Economic Information in an Unapproved Use Dossier is more straightforward than it is for an Unapproved Product Dossier. This is because the manufacturer already has a known price for the approved product. The known price of the approved product should be included in Unapproved Use Dossiers.2
However, there may be times when the price of an unapproved use of an approved product may be different than the price of an approved use of the same approved product. For example, the unapproved use of an approved product may occur in a different patient population and/or have a different formulation that requires a different route of administration than the approved use of the approved product. Per Version 4.1 of the AMCP Format, any potential changes in cost between the unapproved use and the approved use(s) of the product should be mentioned.2 For reasons similar to those described earlier, it may not be possible to construct economic models prior to the approval of an unapproved use of an approved product by the FDA.2
Relationship to the FDA Guidance on Communications by Firms to Payors Regarding Unapproved Products and Unapproved Uses of Approved/Cleared Products
Version 4.1 of the AMCP Format was created in response to the Final FDA Guidance on Drug and Device Manufacturer Communications With Payors, Formulary Committees, and Similar Entities that was published in June 2018.2-4 In fact, in the sections of the AMCP Format that describe Unapproved Product Dossiers and Unapproved Use Dossiers, the AMCP Format specifies which sections of the dossiers arise from the aforementioned FDA Guidance and which ones are AMCP Format recommendations.2 While the FDA Guidance does not specifically mention unmet need, treatment guidelines, or economic models, it does mention information on product pricing and the manufacturer’s ability to provide a “factual presentation of results” related to both placebo and active controls included in trials examining the unapproved product or unapproved use of interest.3 The FDA Guidance also provides some useful examples that highlight some of the differences between providing a “factual presentation of results” from a clinical trial and making a characterization or conclusion about the safety or effectiveness of an unapproved product or an unapproved use.3
Other Considerations for Manufacturers
When developing Unapproved Product Dossiers and Unapproved Use Dossiers, a key factor that must be considered is the time needed for their review and approval. The information in these dossiers is often desired by HCDMs six to 24 months prior to FDA approval,2 so some manufacturers may need to revise and/or optimize their current internal review and approval processes to account for these new types of dossiers, especially if they wish to provide them proactively.
The manufacturer should also think about when to update their dossier, if applicable. In the past, Approved Product Dossiers have been updated in response to a new indication, a new clinical trial, new recommendations from treatment guidelines, and/or the arrival of new competitors to market, among other things. If the initial version of an Unapproved Product Dossier or an Unapproved Use Dossier is completed far ahead of FDA approval, it may be valuable to update the dossier again prior to approval if data from an ongoing clinical trial is published, there is a change in the FDA review time, or information on patient support programs becomes available. For all three dossier types, the decision of whether or not to proceed with dossier updates is up to the manufacturer.2
Conclusions
Version 4.1 of the AMCP Format provides manufactures with guidance on generating a trio of dossiers that can help support a product throughout its lifecycle.2 Even though manufacturers are not required to develop Unapproved Product Dossiers or Unapproved Use Dossiers, they can be useful tools for facilitating communications with HCDMs. We recommend that manufacturers review the options offered by Version 4.1 of the AMCP Format and consider which type of dossier will best meet their needs. Developing an Unapproved Product Dossier or Unapproved Use Dossier not only provides the manufacturer with an opportunity to prepare for the launch of new products and new indications, but also helps HCDMs plan for future FDA approvals.
References
- AMCP. AMCP Format for Formulary Submissions – Guidance on Submission of Pre-approval and Post-approval Clinical and Economic Information and Evidence, Version 4.1. December 23, 2019. Available at: https://www.amcp.org/Resource-Center/format-formulary-submissions/AMCP-Format-for-Formulary-Submissions-4.1. Accessed January 29, 2020.
- AMCP. The AMCP Format for formulary submissions (version 4.1). Guidance on Submission of Pre-approval and Post-approval Clinical and Economic Information and Evidence. December 23, 2019. Available at: https://www.amcp.org/sites/default/files/2019-12/AMCP_Format%204.1_1219_final.pdf. Accessed December 26, 2019.
- FDA. Drug and Device Manufacturer Communications With Payors, Formulary Committees, and Similar Entities — Questions and Answers Guidance for Industry and Review Staff. June 2018. Available at: https://www.fda.gov/media/102683/download. Accessed January 29, 2020.
- AMCP. AMCP Format v4.1: New Guidance on Evidence Requirements for Unapproved Products and Unapproved Uses. January 23, 2020. Available at: https://www.amcp.org/Resource-Center/formulary-utilization-management/amcp-format-v41-new-guidance-evidence-requirements. Accessed January 29, 2020.
For more information, please contact us.