SPRING 2020, THE EVIDENCE FORUM, WHITE PAPER
 Kevin Marsh, PhD Executive Director Commercial Strategy and New Product Development Patient-Centered Research Evidera |  Jessica Griffiths, MEnt Senior Consultant Market Access ConsultingEvidera |  Caitlin Thomas, MSc Research Associate Patient-Centered ResearchEvidera |
Overview
There has been a lot of recent discussion about the potential role of patient preference (PP) data in support of reimbursement decisions. In January 2020, the National Institute for Health and Care Excellence (NICE) published a paper1 that provided more detail on the use of patient preference data. They emphasised their perspective on the importance of PP data and clarified how this can be used by their committees. This article summarizes the key takeaways from NICE’s publication and what this means for sponsors’ evidence generation strategies.
Introduction
PP data quantifies how patients make trade-offs involved in treatment decisions. Decision makers are increasingly interested in using quantitative PP data to support their decisions. For instance, the US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) encourages manufacturers to submit PP data to support its benefit-risk assessment.2 Health technology assessment (HTA) often also involves the use of quantitative preference data. However, HTA agencies have tended to use general population preferences to estimate utility inputs for cost-effectiveness analysis.3 While patient input is sought, it has often been in the form of qualitative insights on the burden of the disease, submissions from patient advocacy groups, or patient representatives being members of decision-making committees.4 There has traditionally been little or no role in HTA for quantitative PP data.
However, agencies such as Sweden’s Dental and Pharmaceutical Benefits Agency (TLV) and Germany’s Institute for Quality and Efficiency in Health Care (IQWiG) have identified formal roles for PP data in their methods guides. Other HTA agencies have more recently also shown an interest in PP data, initiating consultations and pilots to explore how this data might support their decision making.5,6 Notably, in 2019 NICE provided its first scientific advice on PP study design, specifically on how PP data might support the selection of endpoints in a COPD clinical study.5,6 However, questions still remain about how PP data can be used in HTA. In its recent publication,1 NICE begins to answer some of those questions and confirms that the use of PP data is one of NICE’s nine priority research topics. Three main uses of PP studies are identified:
- Clinical trial endpoint selection
- Informing benefit-risk assessments for regulatory approval
- Supporting reimbursement decisions
This article focuses on the use of PP data to support clinical trial endpoint selection and reimbursement decisions. What does NICE’s article say about this use of PP data? What questions still remain? And what does this mean for sponsors’ evidence generation strategies?
Why Does NICE Think There is a Benefit to Conducting a PP Study?
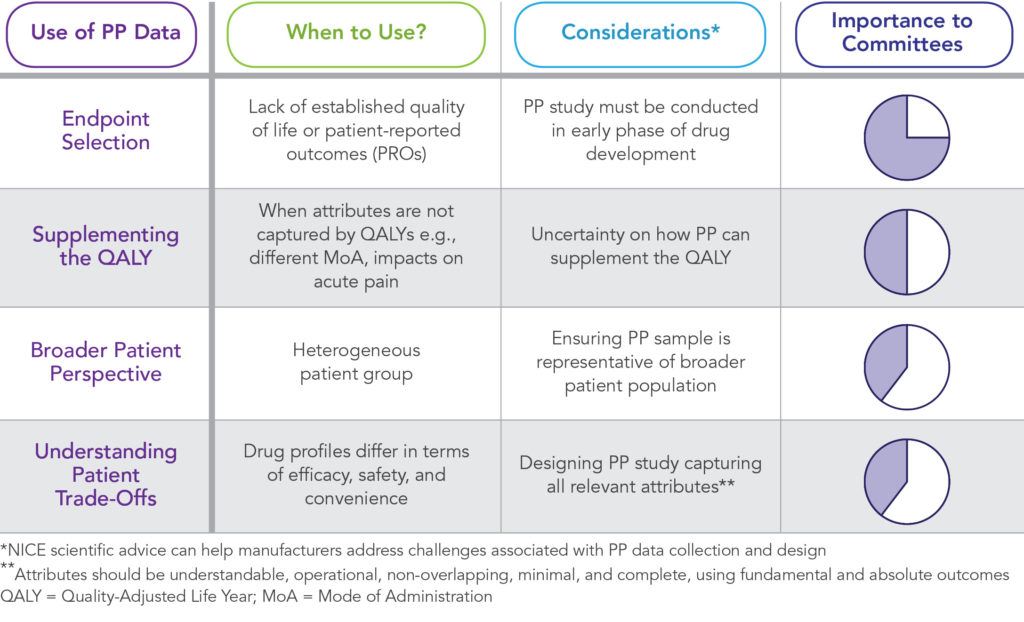
NICE’s publication identifies four ways in which PP data can support their committees.
Ensuring a Representative Picture
NICE currently captures the views and experiences of patients through several routes. These include having lay members on NICE committees and patient organizations and patient experts providing written evidence and attending committee meetings to share their experiences of the condition and, if possible, the treatment being considered. However, NICE has acknowledged that there are limitations to the current approach for ensuring patient input into recommendations. Specifically, only a small subset of patients’ opinions are included and not the wider patient population, which raises concerns that the input provided may not be representative. NICE suggests that PP data may provide a way to overcome this concern, especially if the sample is representative of the broader patient population. Furthermore, PP studies can provide insight on how preferences vary between subsets of patients.
NICE has acknowledged that there are limitations to the current approach for ensuring patient input into recommendations.
Understanding How Patients Make Trade-Offs
Where therapies have quite different profiles, in terms of efficacy, safety, and convenience, PP data can help committees understand how patients make trade-offs when choosing between such treatments. NICE illustrates this with an example of cancer treatments, where chemotherapy, radiation therapy, and immunotherapy differ in modes and ease of administration, effectiveness, and the risk of serious side effects. In such cases, a PP study could provide important insights to a committee on how patients with cancer would make trade-offs between the different treatment options and the probability that patients would prefer one treatment over another.
Supplementing the QALY
NICE does not currently envisage PP data replacing the current method for calculating the incremental cost-effectiveness ratio (ICER) or being used to justify reimbursement of a drug that is not clinically or cost effective. However, they acknowledge that PP studies can supplement ICERs where attributes that are relevant to patients are not captured by quality-adjusted life years (QALYs). For instance, PP data can help committees understand the value that patients place on changes in mode of administration (MoA).
Justifying Endpoint Selection
PP studies can identify the endpoints that matter most to people living with the condition and therefore should be included in clinical trials. NICE’s scientific advice team produced its first ever guidance on a COPD PP study in February 2019. Input was received to improve the design of the COPD patient preference study, inform evidence generation strategy, and collect certain outcome data alongside PP to help in correlating the PP results with current NICE processes for evaluating new treatments.1
Remaining Questions – Precisely How Will NICE Use PP Data?
NICE’s publication provides valuable insight into how PP data can support their committees. However, there remains uncertainty as to precisely how NICE committees will use PP data. For instance, how will patient preferences for new modes of administration inform committee’s decisions? NICE acknowledges that such process utilities (i.e., utility can be affected by the process of treatment, not just the outcomes of treatment) are not captured by the QALY and that PP studies can capture these and thus supplement the QALY. How exactly PP data can supplement the QALY is not clear.
Summary of NICE’s Use of Patient Preference (PP) Data
It is important that sponsors seek scientific advice from NICE on methods for collecting PP data and the proposed use of the data.
Should estimates of process utility generated using PP data be incorporated into an ICER scenario analysis? If so, how should this be done? PP data can be used to express process changes in equivalent changes in health outcomes that can be captured by the QALY. For instance, the improvement in life expectancy that will give the same utility as changing mode of administration. Can these equivalent changes be added into the QALY calculation?
How does NICE reconcile the use of PP data with the priority given to general population preference data in its reference case? Can the above approach – estimating changes in terms of equivalent changes in life expectancy – be interpreted as capturing patient experience, which are still valued in the same way as other measures of patient preferences, in a manner consistent with the NICE reference case?
If the insight from PP data should not be used within scenario analysis of the ICER calculation, how should committees incorporate this data into their decisions? NICE has stipulated that PP data cannot be used to justify the reimbursement of a treatment that is not cost-effective. Could a strong patient preference, for an attribute of a treatment not captured by the QALY, be evidence of uncertainty in the utility estimates included in the cost-effectiveness analysis, and thus a justification for adopting a different cost-effectiveness threshold?
Conclusion
NICE’s publication confirms the importance of PP data to their committees. This is encouraging for sponsors who see patients’ preferences as an important part of their value messages and provides support for them to include PP studies in their evidence generation strategy. However, inevitably there are still questions about precisely how this data will impact reimbursement decisions. Given this uncertainty, it is important that sponsors seek scientific advice from NICE on methods for collecting PP data and the proposed use of the data.
References
- Bouvy JC, Cowie L, Lovett R, et al. Use of Patient Preference Studies in HTA Decision Making: A NICE Perspective. Patient. 2020 Jan 16. doi: 10.1007/s40271-019-00408-4. [Epub ahead of print]
- US Food and Drug Administration (FDA). Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling: Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Available at: https://www.fda.gov/media/92593/download. Accessed March 7, 2020.
- National Institute for Health and Care Excellence (NICE). Guide to the Methods of Technology Appraisal 2013. Available at: https://www.nice.org.uk/process/pmg9/chapter/foreword. Accessed March 7, 2020.
- Health Technology Assessment International (HTAi). Good Practice Examples of Patient and Public Involvement in Health Technology Assessment. Available at: https://htai.org/wp-content/uploads/2018/02/Good_Practice_Examples_Feb_2015.pdf. Accessed March 7, 2020.
- Patalano F, Gutzwiller FS, Shah B, Kumari C, Cook NS. Gathering Structured Patient Insight to Drive the PRO Strategy in COPD: Patient-Centric Drug Development from Theory to Practice. Adv Ther. 2020 Jan;37(1):17-26. doi: 10.1007/s12325-019-01134-x. Epub 2019 Nov 9.
- National Institute for Health and Care Excellence (NICE). NICE Provides First Scientific Advice on Patient Preference Study Design. Available at: https://www.nice.org.uk/news/article/nice-provides-first-scientific-advice-on-patient-preference-study-design. Accessed March 7, 2020.
For more information, please contact us.