FALL 2019, THE EVIDENCE FORUM, WHITE PAPER
 Mariah Baltezegar, MBA Executive Director, Head of PPA Virtual Trials Real-World Evidence Evidera |  Debra Schaumberg, SCD, OD, MPH Vice President, Scientific Affairs Real-World Evidence Evidera |
There is increasing recognition of the need for more fit-for-purpose evidence in development of therapeutics (drug, device, and digital). In the US, in response to the 21st Century Cures Act, the US Food and Drug Administration (FDA) has developed a framework for evaluating real-world evidence (RWE) to support approvals of new indications for previously approved therapeutics and address post-approval study requirements. The collection of RWE is enabled by virtual trials, or decentralized approaches to patient identification and data collection. To inform this approach, we must first understand who the stakeholders are and what their needs are as well as begin speaking the same language around virtual trials; there is no widespread consensus on terminology. Understanding these foundational needs and aligning on definition enables early planning to incorporate such strategies.
Understanding the Needs
Reaching More Patients
Strict inclusion/exclusion criteria and research center-based infrastructure do not serve patients who live remotely, lack transportation, or lead busy lives. This separation of infrastructure makes reaching a heterogenous population challenging. Generally, clinical care facilities do not conduct research and research facilities are either commercial research centers or large, academic teaching centers. Patients who use providers that exist outside of those facilities generally receive existing medical care rather than participate in research. To ensure we are capturing data from a representative sample of patients, we must find ways to reach a wider group of patients. It stands to reason, if patients in the research cohort are more homogeneous than the patient population that would be likely to receive the approved therapy, their data may not be generalizable to the greater population. An inherent tradeoff arises between randomized control design choices aimed at enhancing internal validity with those more pragmatic choices that would aid generalizability. For example, registration trials increasingly tend to enroll relatively small samples of highly selected patients at sites with experienced investigators under ideal conditions, collecting large amounts of very specific data that are often not a routine part of clinical care.
Almost 15% to 20% of trials do not enroll a single patient.1 To continue to evolve the development of fit-for-purpose evidence to inform the real-world use of approved therapeutics, we must make research more accessible. Depending on the research question, a patient may be happy to complete a patient-reported outcome (PRO) or telemedicine visit in their home or at work but not willing to go to a brick and mortar site location to perform the same activities. In this way, we must weigh the burden of participation versus the value of the information. In the United States, 70% of potential patients live over two hours away from the nearest traditional study site,2 which limits participation and leads to higher potential for subject dropout as patients can incur costs and lost time from work associated with traveling to the study site. As virtual trials aim to reduce or eliminate site visits by bringing the trial closer to a patient’s home, more patients have the potential to participate.
Decreasing Burden
Over time, traditional randomized trial protocols have become increasingly more complex. To increase participation and retention, we must decrease the burden of participation for patients and their caregivers. Many clinical trials still rely on 1990s-era processes, and many R&D functions have yet to fully leverage real-world evidence (RWE), genomics information, and emerging data sources such as the Internet of Things (IoT), wearables, mobile apps, and more.2 The use of digital technologies such as eRecruitment, eConsent, ePROs, wearables, and collection of data directly through patients’ electronic medical records, which many virtual trials also employ, allow patients to integrate a trial more or less seamlessly into their lives, therefore reducing burden and decreasing dropout rate. The average dropout rate from trial protocols is 30% based on research by both the Tufts Center for the Study of Drug Development and Forte Research3 and approximately 40% of patients do not end up adhering to trial protocols. This can impact the outcome of a trial and may introduce bias in the assessment of efficacy and safety. These technologies can also help with protocol compliance, as many have the capacity to proactively remind patients to follow a study’s protocol.
Reducing the cost of therapy development is also another key need, though it is early in the lifecycle of virtualization to say where cost savings may be realized. Virtualizing trials can theoretically save time and resources by reducing the number of investigators and sites. The fewer sites a trial utilizes, the lower costs tend to be. Investigator fees are responsible for 40% to 60% of a trial’s budget,4 paying sites for patient visits costs between $3,000 and $7,000 per visit,5 and site activation and management can make up an additional 25% to 30%.4 What we do know is that a virtual approach enables the conduct of large-scale studies that are otherwise cost prohibitive, allowing for fewer sites or even no sites depending on the research question being asked and the types of assessments needed. As virtualization continues to gain momentum and mature, we anticipate more proof points will emerge regarding areas of cost savings.
As virtual study models center around placing patients at the center of a trial and allowing them to participate more easily, they can easily be applied to enable pragmatic trials to collect rich, real-world data.
Putting the Patient at the Center
 A renewed focus on patients and their involvement in healthcare, treatment decisions, and increasingly in designing research is also driving discussions of the role of RWE and pragmatic trials. As virtual study models center around placing patients at the center of a trial and allowing them to participate more easily, they can easily be applied to enable pragmatic trials to collect rich, real-world data. Pragmatic trials draw on the substantial methodological, bias-reducing advantages of random allocation of health interventions combined with the real-world setting of an observational study and naturally lend themselves to virtual approaches. A burgeoning selection of patient/physiological monitoring devices with the potential to provide real-time data on important indicators is an emerging area of innovation with likely applications in the pragmatic trial setting. For certain indications, physiological monitoring may be highly predictive of a clinically relevant endpoint, and real-time collection of symptom scores is another potential application. Regulatory guidance on the use of mobile apps for reporting adverse drug reactions (ADRs) and use of social media is under development.6 To fully realize the value that can be added through more widespread conduct of pragmatic trials, the field must realize a paradigm shift to incorporate data and operational platforms that can capitalize on data capture through electronic health records (EHRs), registries, patient-reported outcomes (PROs), etc., and enrollment infrastructures within integrated health systems. Already gaining traction in the peri- and post-approval time period, moving forward, more pragmatic elements will begin to be introduced earlier, during the formulation of the clinical development plan.
A renewed focus on patients and their involvement in healthcare, treatment decisions, and increasingly in designing research is also driving discussions of the role of RWE and pragmatic trials. As virtual study models center around placing patients at the center of a trial and allowing them to participate more easily, they can easily be applied to enable pragmatic trials to collect rich, real-world data. Pragmatic trials draw on the substantial methodological, bias-reducing advantages of random allocation of health interventions combined with the real-world setting of an observational study and naturally lend themselves to virtual approaches. A burgeoning selection of patient/physiological monitoring devices with the potential to provide real-time data on important indicators is an emerging area of innovation with likely applications in the pragmatic trial setting. For certain indications, physiological monitoring may be highly predictive of a clinically relevant endpoint, and real-time collection of symptom scores is another potential application. Regulatory guidance on the use of mobile apps for reporting adverse drug reactions (ADRs) and use of social media is under development.6 To fully realize the value that can be added through more widespread conduct of pragmatic trials, the field must realize a paradigm shift to incorporate data and operational platforms that can capitalize on data capture through electronic health records (EHRs), registries, patient-reported outcomes (PROs), etc., and enrollment infrastructures within integrated health systems. Already gaining traction in the peri- and post-approval time period, moving forward, more pragmatic elements will begin to be introduced earlier, during the formulation of the clinical development plan.
The Many Names of “Virtual” Trials
Although there are examples of virtual trials that date back several decades,7,8 the incorporation of virtual trials into commercial therapeutic development and product lifecycle management is an emerging concept and there is no uniform agreement on definitions. Although non-interventional studies are not typically considered a “trial,” the term has been applied in the context of both interventional as well as non-interventional studies.
The most common terms used to define this paradigm in which studies are conducted either partially or entirely remotely include:
- Virtual trials. An umbrella term used to describe collecting data from patients in their local healthcare environment versus requiring them to go to a clinical research site or other brick and mortar location. This can be accomplished with or without technology and moves research away from the traditional site visit model to a more disseminated model where patients can participate from their homes and nearby surroundings. Virtual trials have been conducted for decades and now, with the advent of enabling technologies, are often digitally enabled.
- Digitally enabled trials. These are studies that use digital technologies to enhance the efficiency of a trial, including well-established technologies such as electronic clinical outcome assessments (eCOAs) or electronic PROs (ePROs) to newer technologies such as telemedicine and wearables. Studies have been incorporating certain digitally enabled technologies for decades. The key now is to integrate multiple technology solutions for a trial either with a single vendor or, at minimum, with a solid solution for integration of data from multiple technology partners.
- Decentralized trials. This is a term describing the movement away from the site-based trial model, in which all trial activities are centered on the site, to more of a model where patients are the primary focus. This term is used by regulatory agencies including the FDA and the Medicines and Healthcare products Regulatory Agency (MHRA) and it has been reported that the FDA has created a working group on the topic of decentralized trials that will be charged with outlining standards for this new space.8
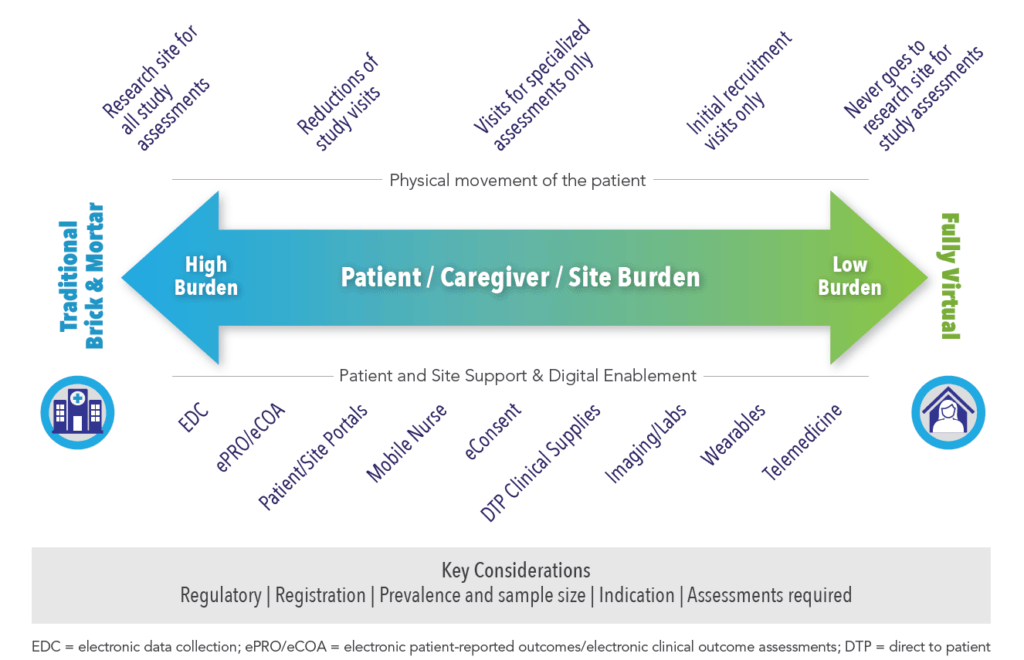
There are three dimensions to consider when assessing virtualization, including burden on the patient, caregiver, or site; physical movement of the patient; and, support and digital enablement (See Figure 1).
Not only does the pharmaceutical industry recognize the need to transform RWE collection, but regulators do as well. In January 2019, the commissioner of the FDA at that time, Scott Gottlieb, MD, shared his goals including supporting seamless integration of digital technologies in clinical trials and bringing clinical trials directly to the patient. Virtual trials enable efficient collection of RWE by bringing the trial to the patient instead of the patient to the trial. Whether we are referring to truly virtual trials, decentralized trials, or digitally enabled trials, each has its merits to facilitate right-sized RWE collection to support the development and commercialization of therapeutics.
References
- The Numbers Game: Boosting Clinical Trial Enrollment. Pharmaceutical Technology, February 5, 2014, Available at: http://www.pharmaceutical-technology.com/features/featurethe-numbers-game-boosting-clinical-trial-enrolment-4171654/. Accessed September 1, 2019.
- Deloitte’s Life Sciences & Health Care Blog. A View from the Center. Available at: https://blogs.deloitte.com/centerforhealthsolutions/digital-rd-four-ways-to-maximize-patient-engagement-in-clinical-trials/. Accessed September 1, 2019.
- Forte. Lopienski K. Retention in Clinical Trials – Keeping Patients on Protocols. June 2015. Available at: https://forteresearch.com/news/infographic/infographic-retention-inclinical-trials-keeping-patients-on-protocols/. Accessed September 1, 2019.
- Araujo DS. Measuring the Financial Impact of Remote (Digital) Clinical Trials. Clinical Leader. Guest Column, January 20, 2019. Available at: https://www.clinicalleader.com/doc/measuring-the-financial-impact-of-remote-digital-clinical-trials-0001. Accessed September 1, 2019.
- Fast Company. The Search for New Drugs is Coming to Your House. Available at: https://www.fastcompany.com/90229910/virtual-clinical-trials-are-bringing-drugdevelopment-home. Accessed September 1, 2019.
- Schaumberg DA, Mihos M, Payne K, Chen D. The Drive Towards Pragmatism in Randomized Trials: Are We There Yet? The Evidence Forum. November 2017. Available at: https://www.evidera.com/wp-content/uploads/2017/10/The-Evidence-Forum-2017-November.pdf. Accessed September 2, 2019.
- Buring JE, Hennekens CH. Cost and Efficiency in Clinical Trials: The U.S. Physicians’ Health Study. Stat Med. 1990 Jan-Feb;9(1-2):29-33.
- US Food and Drug Administration. Breaking Down Barriers Between Clinical Trials and Clinical Care: Incorporating Real World Evidence into Regulatory Decision Making. January 28, 2019. Available at: https://www.fda.gov/news-events/speeches-fda-officials/breaking-down-barriers-between-clinical-trials-and-clinical-care-incorporatingreal-world-evidence. Accessed September 2, 2019.
For more information, please contact us.