FALL 2019, THE EVIDENCE FORUM, WHITE PAPER
 Brenda Garrison Senior Director Project Management Therapeutic Area Lead – Hematology/Oncology Peri- and Post-Approval Interventional Studies Evidera |  Kristin Kluthe, PMP Director Project Management Peri- and Post-Approval Interventional Studies Evidera |  Ethel Pilati, MSc Associate Director Project Management Peri- and Post-Approval Interventional Studies Evidera |
In recent years, traditional communication mechanisms regarding clinical studies have changed significantly. Patients no longer take a back seat in healthcare decision making; therefore, strategies to attract and engage them must evolve to meet their expectations. The era of patient centricity is here, and patient perspectives must be taken into account every step of the way through product development and commercialization.
The shift toward patient centricity is partly due to advancements in technology and communication. Additionally, collaboration with patient advocacy groups and a surge in social media platforms provide patients and caregivers with more options to find the best study to meet their needs throughout their disease journey. Furthermore, with an ever-increasing number of clinical studies around the world, competition for enrollment continues to be a challenge.
With patients taking a more proactive role in their own healthcare decisions, there is an increasing need to adopt innovative approaches for patient recruitment and retention that highlight the benefits of clinical research and differentiate your clinical study from other choices. Study branding – establishing a unique name and visual identity – can be a highly effective tool to increase study awareness, interest, and immediate brand recognition for site personnel, patients, and caregivers.
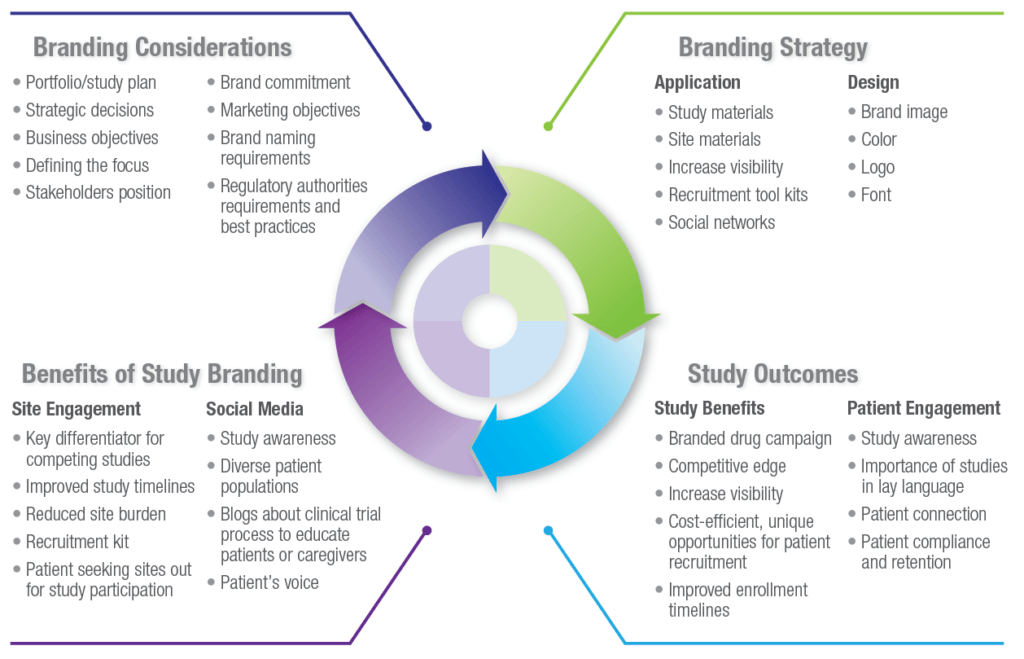
Branding Considerations
Once a company has decided there is benefit in branding a clinical study, there are many factors and stakeholders to consider before moving forward.
- What does the current and emerging competitive study landscape look like?
- How many other studies are being conducted in the same space?
- What are the specific objectives for branding the study?
- Is the intention to use the study branding in marketing activities once the product has been launched?
- Who is the target audience or audiences?
A powerful and successful approach to study branding should start at the study design phase and it is important at this point to consider several factors before moving forward.
- Patient population. Pay attention to your audience and what speaks to them specifically. Is the study focusing on the pediatric or adult population? Women or men? Predominantly low or high income? Different patient segments will relate to branding in various ways so be sure you are considering your audience. A good resource for input in this area could be patient advocacy groups or other groups that could help identify potential trigger words or images that may have a negative effect on the patient population.
- Geographic concerns. Certain words, phrases, and images may have different meanings depending on the location of your target audience, specifically from country to country, but also potentially within countries. For example, a colloquial term or play on words quite familiar in one part of the United States may fall flat in other areas of the country. Do your homework and test branding elements across various geographic regions before finalizing your branding.
- External considerations. As with any clinical study communications, branding elements must be regulatory compliant; it is, therefore, important to confirm any branding name or graphics meet necessary guidelines and can stand up to review board scrutiny.
Branding Strategy
As the creative phase begins it is important to remember that any designs and/or messaging needs to connect with patients and their families or caregivers. The selection of an impactful study name or acronym needs to relate to the patient population and the inclusion of appealing visual aspects, such as graphics, photos, illustrations, etc., should be used to help simplify the message and grab the attention of your audience.
Many study and drug descriptions use scientific jargon that can make it challenging for patients to understand. Developing recruitment materials with a patient audience in mind, speaking in patient-friendly terminology, and clearly illustrating the benefits to the patient are more likely to attract participants and may have a significant impact on enrollment timelines.
Branded materials must be high quality, professional, and appeal to the targeted audience. Attention should be paid to design factors such as color, fonts, images, tone, consistency, and how each branded piece will be used. It is important to use a designer who is experienced in these elements and can produce a full spectrum package of content. A professional designer can also ensure that research previously done on patient populations, geographic concerns, and external considerations is considered during the development of these materials. Patients and caregivers need to have confidence that the study they are entering is safe and beneficial, and high quality, professional materials are an important aspect of building that image. In the internet era patients can easily access information about drug competitors, drug safety, and efficacy profiles; therefore, any communications or information shared on the study should inspire the confidence patients require to comfortably select your study if it best fits their needs.
… it is important to remember that any designs and/or messaging need to connect with patients and their families or caregivers. The selection of an impactful study name or acronym needs to relate to the patient population …
Conclusion
Study branding is a critical aspect of study planning and can have significant impact on the success of a study. Early discussions about the benefit of study branding, along with key input from patient advocacy groups and alignment with regulatory authorities and ethics committees, will raise awareness and reinforce the study through the consistent use of graphics, images, and visuals via numerous forms of communication. While this article focuses on key considerations in a decision to brand and how to best connect with patients for recruitment and retention purposes, there are a myriad of other considerations and benefits that could arise from branding your study (See Figure 1). As study options increase in certain therapeutic areas and companies look to decrease timelines and increase brand recognition, expect study branding to grow as companies seek a competitive advantage to achieve product success. Not all studies need to be branded, but it is highly recommended that you consider the option when designing your clinical study.
Special thanks to Christina Kirkpatrick, Senior Account Director, Business Development, PPD, who contributed to this article.
For more information, please contact us.