SPRING 2021, THE EVIDENCE FORUM, WHITE PAPER
 Margaret Richards, PhD, MPH Senior Research Leader Real-World Evidence Evidera, a PPD business |  Yin Hong, PhD Research Associate Real-World Evidence Evidera, a PPD business |  Kenneth Butz, MSc Director, Regulatory Affairs Regulatory Technical Advisors PPD |
In Vitro Diagnostic EUAs for COVID-19
Since the US Department of Health and Human Services Secretary declared COVID-19 a public health emergency on February 4, 2020, the US Food and Drug Administration (FDA) has issued numerous Emergency Use Authorizations (EUAs) for in vitro diagnostic devices (IVDs) to detect various targets of current or past COVID-19 infection.
An EUA is one of several tools the FDA has used to quickly make certain medical products available during the COVID-19 pandemic. In emergencies, the FDA can issue an EUA to provide access to medical products that may be used when there are no adequate, approved, or available options.1 The EUA process is different than an FDA approval or clearance. Under an EUA, the FDA makes a product available to the public based on the best available evidence, without waiting for all the evidence that would be needed for full approval.1 EUAs remain in effect until the emergency declaration ends.
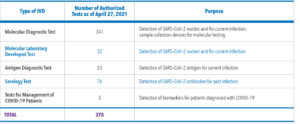
The amount of EUA activity for IVDs in the past year is stunning, as shown in Table 1.
The greatest number of IVD EUAs are associated with molecular diagnostic tests (n=241). We chose to take a closer look at antigen diagnostic tests, however, because they hold the most public health promise in terms of speed, ease of administration, reasonable sensitivity and specificity, and cost.
Antigen Diagnostic Tests
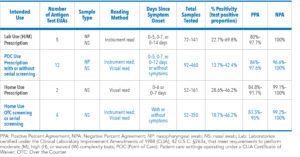
Antigen diagnostic tests identify the SARS-CoV-2 nucleocapsid protein antigen. Currently, the molecular diagnostic test using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) remains the “gold standard” for the diagnosis of COVID-19 due to its high sensitivity and specificity to detect viral RNA.3 However, RT-PCRs often require longer turnaround times and must be processed by trained laboratory staff with higher associated costs for the test kit and equipment.4 Antigen diagnostic tests, which require minimal training and equipment, have faster processing times and lower costs. Table 2 compares the antigen diagnostic test to the standard RT-PCR.
A total of 23 antigen diagnostic tests have been authorized by the FDA as of April 27, 2021 (See Table 3). Samples from nasopharyngeal or nasal swabs can be collected and processed by healthcare providers for point-of-care (POC) use or self-/other-administration in home use. Some antigen tests require a prescription and symptoms, whereas others are made available over-the-counter (OTC) with symptoms optional. Some tests are for adults only whereas others can be used for ages two and older.
EUA for Antigen Diagnostic Test
For antigen diagnostic test developers requesting an EUA, the FDA recommends several validation studies to determine the test’s clinical and analytical performance. The FDA website has two templates, one for an antigen diagnostic test for laboratory and POC use and one for home use,2 that include some of the validation studies needed for analytical performance (See Figure 1). For clinical performance, a usability study is recommended for the POC claim to demonstrate that healthcare providers can perform the test from the instructions given in the test kit. This is also recommended for home use to demonstrate that an individual can perform the test accurately, either self-collected or other-collected, depending on the intended use.
Clinical evaluation is done to compare the performance of the antigen test versus a comparator RT-PCR test authorized by the FDA.7 Performance is assessed as Positive Percent Agreement (PPA) and Negative Percent Agreement (NPA), percentages that are similar in concept to sensitivity and specificity, respectively. As there is no current reference standard available, PPA and NPA are used instead of sensitivity and specificity because the latter assume a reference standard.
A PPA of ≥ 80% is required for laboratory-based POC and home use tests that are prescription only. For OTC home use, a PPA ≥ 90% and an NPA ≥ 99% are required for both asymptomatic and symptomatic individuals.
Challenges
In general, antigen tests have high specificity (NPA), but relatively moderate sensitivity (PPA) compared to an RT-PCR (See Table 2). The sensitivity of antigen tests drops in samples with RT-PCR cycle threshold (Ct) values > 30, which are samples with medium to low viral loads.8 A Ct value is the cycle of amplification at which the fluorescence crosses the threshold to become positive and viral load and Ct threshold values are inversely correlated. Simply put, the higher the viral load the lower the Ct value and vice versa. Although more data are required, higher viral load is thought to be related to higher transmissibility9 and risk of intubation and mortality.10 An antigen test may identify individuals with higher viral load who are most likely to infect others. Viral loads correlate well with date of diagnosis and/or symptom onset; they are the highest within 1-5 days of infection and decline thereafter.9
Interestingly and importantly, viral load does not seem to correlate with any one COVID-19 symptom or symptom constellation. Those who are asymptomatic but have a positive test can nonetheless have a high viral load (and transmit disease), which is one of the characteristics of COVID-19 that has made it difficult to manage. As the pandemic begins to wane globally, rapid antigen tests on both symptomatic and asymptomatic persons for screening purposes could be particularly useful.
Future Trends
Although an antigen diagnostic test is not as sensitive as an RT-PCR, it can be very useful from a public health perspective. Situations of public health import include:
- Persons with limited access to a standard RT-PCR
- Individuals not meeting RT-PCR testing criteria
- Home-use/screening (when COVID-19 exposure is suspected or known, especially with underlying conditions or susceptibility)
- Community settings such as universities or workspaces where large numbers of people gather regularly and need to be tested often
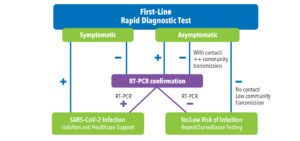
One study has shown that frequent mass testing using rapid tests as part of a screening program might be more cost-effective than a standard testing approach.11 To date, only four rapid antigen tests and two rapid molecular tests have received an EUA for OTC use (symptomatic and asymptomatic individuals, screening, or serial screening). Various testing algorithms have been proposed by health authorities using rapid tests as first-line screening under certain conditions and, depending on the screening result, branching out into different decisions involving RT-PCR test confirmation, isolation, surveillance testing, etc.
Figure 2 shows one test flow. Other test flows have been devised by regulatory and health authority agencies around the globe.
The beauty of a rapid diagnostic first-line test is the ability to scale mass testing with low cost and rapid response. An approach that involves RT-PCR testing first-line is potentially expensive and can cause delays. The fact that these rapid tests have moderate sensitivity and high specificity is an advantage. There are few false positives (due to high specificity) and more false negatives (due to moderate sensitivity), but the latter are assumed to be associated with a lower viral load and thus a less contagious individual. If a false negative rapid test individual is allowed to move about his/her sphere, the chances are that his or her viral load is no longer a threat.
Conclusion
The COVID-19 pandemic has stimulated innovation in COVID-19 IVD assays, treatments, and vaccines, and that progress has brought confusion, disappointment, rapidity, ingenuity, and elegance. There is no doubt that diagnosis, treatment, and prevention will all play key roles in managing the current and any future pandemics. Hopefully, the lessons learned from COVID-19 diagnostic product development will translate to other IVD diagnostics, infectious or otherwise.
References
- US Food and Drug Administration (FDA). Understanding the Regulatory Terminology of Potential Preventions and Treatments for COVID-19. Available at: https://www.fda.gov/consumers/consumer-updates/understanding-regulatory-terminology-potential-preventions-and-treatments-covid-19. Accessed March 11, 2021.
- FDA. In Vitro Diagnostics EUAs. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas. Accessed April 27, 2021.
- Kevadiya BD, Machhi J, Herskovitz J et al. Diagnostics for SARS-CoV-2 Infections. Nat Mater. 2021 Feb 15. doi: 10.1038/s41563-020-00906-z. Online ahead of print.
- Peeling RW, Olliaro PL, Boeras DI, Fongwen N. Scaling up COVID-19 Rapid Antigen Tests: Promises and Challenges. Lancet Infect Dis. 2021 Feb 23;S1473-3099(21)00048-7. doi: 10.1016/S1473-3099(21)00048-7. Online ahead of print.
- Ramdas K, Darzi A, Jain S. ‘Test, Re-test, Re-test’: Using Inaccurate Tests to Greatly Increase the Accuracy of COVID-19 Testing. Nat Med. 2020 Jun;26(6):810-811. doi: 10.1038/s41591-020-0891-7.
- Centers for Disease Control and Prevention (CDC). Interim Guidance for Antigen Testing for SARS-CoV-2. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html. Accessed March 16, 2021.
- FDA. Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/policy-coronavirus-disease-2019-tests-during-public-health-emergency-revised. Accessed March 16, 2021.
- Jefferson T, Spencer EA, Brassey J, Heneghan C. Viral Cultures for COVID-19 Infectious Potential Assessment – A Systematic Review. Clin Infect Dis. 2020 Dec 3;ciaa1764. doi: 10.1093/cid/ciaa1764. Online ahead of print.
- Kawasuji H, Takegoshi Y, Kaneda M et al. Transmissibility of COVID-19 Depends on The Viral Load Around Onset in Adult and Symptomatic Patients. PLoS One. 2020 Dec 9;15(12):e0243597. doi: 10.1371/journal.pone.0243597. eCollection 2020.
- Magleby R, Westblade LF, Trzebucki A et al. Impact of SARS-CoV-2 Viral Load on Risk of Intubation and Mortality Among Hospitalized Patients with Coronavirus Disease 2019. Clin Infect Dis. 2020 Jun 30;ciaa851. doi: 10.1093/cid/ciaa851. Online ahead of print.
- Du Z, Pandey A, Bai Y et al. Comparative Cost-Effectiveness of SARS-CoV-2 Testing Strategies in the USA: A Modelling Study. Lancet Public Health. 2021 Mar;6(3):e184-e191. doi: 10.1016/S2468-2667(21)00002-5. Epub 2021 Feb 4.
- Government of Canada. Interim Guidance on the Use of Rapid Antigen Detection Tests for the Identification of SARS-CoV-2 Infection. Available at: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/use-rapid-antigen-detection-tests.html. Accessed March 16, 2021.
- Infectious Diseases Society of America. IDSA Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/. Accessed March 16, 2021.