SPRING 2021, THE EVIDENCE FORUM, WHITE PAPER
 Kristin Veley, PharmD, MPH Research Scientist and Director REMS and Pregnancy Registries Real-World Evidence Evidera, a PPD business |  Jason Simeone, PhD Senior Research Scientist and Director, US Database Analytics Real-World Evidence Evidera, a PPD business |  Nicole Hurst, MSW Senior Director, Project Management Real-World Evidence Evidera, a PPD business |
Introduction
The exclusion of pregnant and lactating women from pre-approval clinical trials, for COVID-19 vaccines and other products, results in a lack of safety and efficacy information for this population and necessitates post-approval research. Flexible, observational safety studies of pregnant and lactating women, and of their infants, are imperative to ascertain the impact of product exposure and assess the risk of adverse pregnancy, fetal, and infant outcomes. These studies complement pre-approval clinical trial data and add to the body of evidence regarding product safety and effectiveness.
COVID-19 vaccines present a current and salient example of the need for flexible pregnancy safety study design, allowing us to demonstrate the importance of these studies, propose solutions to commonly encountered challenges, and highlight the best practices and benefits associated with various study designs. To meet the need for post-approval research on the safety of COVID-19 vaccines in pregnant and lactating women, several types of real-world study designs can be implemented—all of which meet regulatory standards and supplement existing vaccine surveillance systems.
Current Regulations for Pregnancy Safety Studies in the US and EU
Before we review pregnancy study options, let’s survey the current regulatory landscape related to pregnancy safety studies, both in the United States (US) and in the European Union (EU).
The US:
Food and Drug Administration (FDA) Requirements
The draft guidance released by the FDA in May 20191 increased the rigor of pregnancy safety studies considerably. Now, for products expected to have sufficient pregnancy exposures, the FDA may require sponsors to conduct both:
- A prospective, registry-based observational exposure cohort study comparing the maternal, fetal, and infant outcomes of women exposed to the product during pregnancy to an unexposed control population. Adverse outcomes will be assessed throughout pregnancy. Adverse infant outcomes will be assessed through at least the first year of life, AND
- A retrospective cohort study using claims or electronic health records (EHRs) data, or a case control study to assess adverse pregnancy outcomes in women exposed to the product during pregnancy compared to an unexposed control population.
For products expected to have rare pregnancy exposures, the FDA requires that sponsors conduct:
- A worldwide descriptive study, or a global surveillance program, that collects prospective and retrospective data in women exposed to the product during pregnancy to assess the risk of pregnancy and maternal complications, adverse effects on the developing fetus and neonate, and adverse effects on the infant. Infant outcomes will be assessed through at least the first year of life, and the study will collect information for a minimum of 10 years.
The EU:
European Medicines Agency (EMA) Requirements
Risk Management Plans (RMPs)
In the EU, every authorized product requires a risk management plan (RMP). RMPs should reflect the measures considered necessary to identify, characterize, and minimize a medicinal product’s important risks. For products with anticipated use in women who are or who may become pregnant, the RMP should also include current understanding of safety in pregnancy and/or breastfeeding, along with the likelihood of use of the medicine in women of child-bearing potential, or who are pregnant or breastfeeding.
Post-Authorization Safety Studies (PASS)
Additional pharmacovigilance activities in the form of PASS should be used if/when:
- The use of a product cannot be discontinued due to the disease being treated, when a disorder arises during pregnancy that necessitates treatment, or when changes in treatment during pregnancy are associated with risks for the pregnant woman and/or fetus.
- A potential risk to the child has been suggested by non-clinical data, a signal, or based on the chemical or pharmacological properties of the medicine.
- The medicine is used to treat conditions that commonly occur in women of child-bearing potential.
- Measuring compliance with risk minimization measures (RMM) regarding pregnancy or breastfeeding.
The EU’s current good pharmacovigilance practices (GVP) guidelines for pregnancy studies recommend the following:
- Disease-specific rather than product-specific registries
- Use of existing registries and databases
- Hybrid/ambispective, multi-country study designs
- Prospective enrollment
- Comprehensive inclusion criteria (minimal exclusion criteria)
- Long-term infant follow-up to assess developmental outcomes
- Standardized data collection
- Inclusion of study information in mandated educational materials
The EU currently recommends pregnancy studies to record the following pregnancy outcomes:
- Malformation/anomalies diagnosed in utero, at birth, or at follow-up
- Ectopic pregnancy, molar pregnancy, spontaneous abortion, elective termination, late fetal death, stillbirth, or live birth
- Infant growth, development, illnesses, and hospitalizations
Pregnancy Safety Studies Are on the Rise
There is an encouraging upward trend in the number of pregnancy safety studies conducted in both the US and the EU.
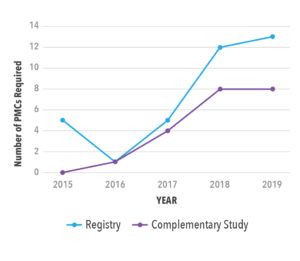
A search of the FDA listing of post-marketing commitments (PMCs)2 revealed a significant increase, from 2015 to 2019, in the number of pregnancy safety requirements mandated by the Center for Drug Evaluation and Research (CDER) for New Drug Applications (NDA) and Biologics License Applications (BLA). This trend applied for both pregnancy registries and complementary studies (e.g., retrospective database studies) (See Figure 1). By 2019, more than 25 percent of approvals required a pregnancy registry, and nearly 20 percent required a complementary study. Complementary studies were always paired with registry studies unless an existing registry (for example, for the class of medications or disease) was already in progress. Pregnancy post-marketing commitments varied by therapeutic area, with products treating autoimmune disorders being most likely to need a pregnancy safety study and oncology and infectious disease products being least likely.
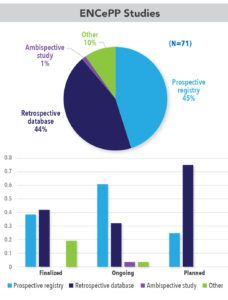
A search of the ENCePP (European Network of Centers for Pharmacoepidemiology and Pharmacovigilance) database produced 71 studies that were conducted among pregnant women and assessed the risk of adverse pregnancy and infant outcomes. Of these, about 10 percent were classified as “other,” as they were case-controlled studies, meta-analyses, or systematic reviews. There was one ambispective study, and the rest were fairly evenly split between prospective and retrospective studies. When stratified by study status—finalized, ongoing, or planned—we noticed a large uptick in the number of retrospective studies in the planning phase (See Figure 2).
We anticipate that these positive trends observed in FDA and ENCePP data will continue, and that we will see an increasing number of pregnancy safety studies implemented to assess product safety in pregnant and/or breastfeeding women.
COVID-19 Vaccines and the Need for Flexible Pregnancy Safety Studies
The recently approved COVID-19 vaccines present a timely and compelling opportunity to design flexible pregnancy safety studies. Evidence has accumulated from a variety of sources indicating that pregnant women are at higher risk of severe COVID-19 infection compared to non-pregnant adults.
A study published in the American Journal of Obstetrics and Gynecology in January of 20213 found that pregnant women are in fact at higher risk of severe disease and mortality compared to non-pregnant adults. This multi-center retrospective cohort study in Washington state compared case fatality rates between pregnant women and similarly aged, non-pregnant adults; maternal and neonatal outcomes were also compared by trimester of infection and disease severity at the time of delivery. The study found that hospitalization and case fatality rates were significantly higher in pregnant women, and that pregnant women with severe COVID-19 infections were at higher risk of pre-term delivery than women who recovered or had mild infections.
The Centers for Disease Control (CDC) and other institutions have published similar findings related to COVID-19 and pregnancy. Based on the accumulation of data, the American College of Obstetricians and Gynecologists (ACOG) recommends that COVID-19 vaccines should be available and administered to pregnant women who wish to be vaccinated, that pregnant women should be free to make their own decision regarding vaccination, and that women should not be denied a vaccine due to their pregnancy status alone.4
ACOG Recommendations for COVID-19 Vaccination in Pregnant Women4
- COVID-19 vaccines should be available and administered to pregnant women who wish to be vaccinated.
- Documentation of a discussion is not required.
- Pregnancy testing prior to vaccination is not required.
- Pregnant women can receive a vaccine in any setting.
- Precautions should be discussed if there was a previous allergic reaction to vaccines or polysorbate.
- If anaphylaxis occurs, the same management is recommended.
- If a fever occurs, administer acetaminophen.
- Encourage participation in V-SAFE after vaccination.
Safety Surveillance Systems for COVID-19 Vaccines in Pregnant Women
The CDC and the EMA have both identified pregnant women as a population of interest relative to COVID-19 vaccinations, and have issued plans and recommendations for further research in this population. Both the US and the EU plan to utilize existing safety surveillance systems to monitor COVID-19 vaccine safety in pregnant women In the US, the CDC plans to leverage Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD), and V-SAFE; in the EU, the EMA encourages collaboration with COVID-19 infectiOn aNd medicineS In preGNancy (CONSIGN), International COVID-19 and Pregnancy Registry (COVI-PREG), and the International Network of Obstetric Survey Systems (INOSS).
Some of these systems existed prior to COVID-19, including VAERS, VSD, Vaccines and Medications in Pregnancy Surveillance System (VAMPSS), and INOSS, while others are COVID-19-specific. V-SAFE is a new, smartphone-based active surveillance program in the US that conducts health checks of vaccine recipients via text message or email. If an important adverse event is reported, a telephone follow-up and a report are completed if appropriate. COVI-PREG and CONSIGN are also COVID-19-specific initiatives. Figure 3 outlines the existing surveillance systems that can be leveraged to monitor COVID-19 vaccine safety in pregnancy.
Approved COVID-19 Vaccines and Planned Pregnancy Safety Studies
In the US, three COVID-19 vaccines are currently approved under the FDA’s Emergency Use Authorization: those manufactured by Pfizer-BioNTech, Moderna, and Johnson & Johnson (Janssen). The FDA approval letters for these vaccines indicate that all three have identical post-marketing requirements related to pregnancy. They must conduct observational studies to evaluate the safety of their vaccines in several populations of interest, including pregnant women, and the FDA further specified that these studies should be conducted in large-scale databases with active comparator groups.
In the EU, four vaccines are currently authorized for use. In addition to the three COVID-19 vaccines approved in the US, the AstraZeneca vaccine is authorized in the EU. Routine safety monitoring for these vaccines includes adverse event reporting via EudraVigilance, which is the system operated by the EMA for tracking suspected product side effects. The EMA also releases monthly safety updates on each authorized vaccine. The RMP for each vaccine includes several studies that either specifically or potentially evaluate pregnancy safety. Several prospective pregnancy registries are planned, and studies using secondary data sources are ongoing or planned for each vaccine.
A Closer Look at Flexible Pregnancy Study Designs
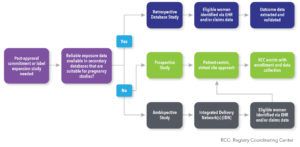
A flexible approach to pregnancy study design encourages participation and facilitates data collection, resulting in greater sample sizes and a more thorough assessment of safety. We’ll consider the common challenges presented by prospective, retrospective, and ambispective pregnancy study designs, and offer practical solutions. There are multiple factors to consider when selecting a study design (See Figure 4).
Prospective Pregnancy Registry Design
Eligible women typically enroll in a prospective registry after they become aware of their pregnancy, and they provide consent and medical releases for their healthcare providers (HCPs) to submit data to the registry. Only data routinely documented in the patient’s chart as part of usual care are collected. Data are typically collected at enrollment, at the end of the second trimester, and at or immediately after the pregnancy outcome. For live-born infants, data collection is continued post delivery, typically at four and twelve months. The pregnant woman herself is typically responsible for answering some initial eligibility questions and providing basic demographic data at enrollment. The majority of the data are collected from the healthcare providers involved in her care, or in the care of her infant, minimizing the burden on the patient.
One of the greatest challenges with prospective studies is recruiting eligible patients. A patient-centric, virtual site approach enables pregnant women to enroll in a prospective registry regardless of their proximity to a study site. Creation of a targeted and customized awareness plan for each registry assists in reaching HCPs and patients in a variety of settings. To encourage enrollment, it is helpful to create a registry website, and to place links to the website on other prominent websites, such as the FDA’s listing of pregnancy registries and sponsor- and product-specific websites.
There are some notable recruitment challenges that are specific to COVID-19 vaccines. Because COVID-19 vaccines are not currently explicitly indicated in pregnancy, there may be a barrier to recruitment. It will be important to use a multi-pronged recruitment strategy to leverage existing data sources to bolster recruitment, and to monitor COVID-19 cases and vaccines in real time. It may also prove challenging to confirm exposure data, due to the variety of settings in which vaccines are offered, and perhaps also the lack of patient awareness of the brand of vaccine that was received. A potential solution may be photo documentation of vaccine records to confirm exposure, or confirmation of exposure through either the patient’s HCP or the vaccine administrator.
Retrospective Pregnancy Registry Design
Retrospective study designs utilize secondary data, which are collected in the usual course of medical care and accessed through de-identified databases that represent large populations of patients. The data are historical and analyses are typically iterative, in which interim analyses are repeated until the set of final analyses. Subsequent analyses can include women who newly qualify for the study based on drug exposure, as well as additional follow-up data on infants. Twelve-month infant follow-up is typically required because not all congenital malformations—which are the standard primary outcome of interest—are diagnosed at birth. The baseline period is used to characterize patients based on demographic and clinical characteristics, as well as healthcare resource utilization, and the pregnancy period is estimated based on gestational age information within diagnosis codes recorded during the pregnancy.
Retrospective Pregnancy Registry Design: Database Considerations
- Pregnant women are identified from claims or EHR database
- When using claims, typically use a closed claims system database for complete view of all covered services
- Must be able to link mother to child in database
- Even in databases where a linkage is available, not all mothers can be linked to their infants
- In the absence of a validated algorithm to identify outcomes, must be able to directly validate outcomes via charts
- Sample size must be considered, but use of newly-approved drugs is expected to be low at initiation of study
There are several challenges unique to retrospective study designs. It can be difficult to select an appropriate comparator, given that comparators may differ by therapeutic area and other approved medications for that indication. Appropriate comparators may include “healthy” controls and women taking medications off-label for that indication. Low sample sizes are a common concern, particularly when a medication, treatment, or vaccine is new. Sample sizes might be increased by adding databases or extending the study period when necessary. While the date of conception or date of last menstrual period is not captured in claims data, the start of pregnancy can be estimated using validated algorithms that use the gestational age information recorded in diagnosis codes during the pregnancy period.
Ambispective Pregnancy Registry Design
An ambispective registry design implements some of the features of both retrospective and prospective registries. The term hybrid is sometimes used to describe this design, but ambispective is more accurate. Ambispective studies use data from large integrated delivery networks (IDNs). Patients are identified via EHRs, which can be supplemented with prospective data collection if needed. The greatest benefit is that data can be captured from multiple sources without having to go directly to that source or site. Because patients who are insured by the IDN are typically incentivized to visit IDN-owned facilities, there is typically reliable identification of care across various healthcare settings.
Ambispective registries can mitigate many of the challenges associated with retrospective and prospective study designs. For example, potentially eligible patients can be identified directly from the EHR data to mitigate recruitment challenges, and data that are not available or poorly captured in the EHRs may be collected directly from patients.
Additional Pregnancy Safety Studies
Lactation Studies
In lactation studies, the objective is to evaluate whether there is a breastmilk transfer of the product from the mother to the infant, to calculate the estimated infant dose, and also to evaluate the safety of the breastfed infants.
Placental Transfer Studies
In placental transfer studies, the objective is to evaluate whether there is a placental transfer of the product from the mother to the infant.
Pharmacokinetic Studies
In pharmacokinetic (PK) studies, the objective is to evaluate whether the physiological changes that occur during pregnancy impact the PK profile of a product.
Other Observational Studies
Other types of observational studies can be conducted for a variety of reasons (e.g., to expand the label or to inform future research).
Regardless of the type of pregnancy safety study, it is critical to design flexible models that encourage patient enrollment. Solutions may include hybrid models with both in-person and remote enrollment, as well as flexible recruitment methods that utilize advertising, social media, and advocacy groups. Streamlined data collection relieves the burden on the patient and the HCP. For example, home health nurses can be used in lactation and/or placental transfer studies, allowing data collection to occur without the patient ever leaving home and without undue burden at the healthcare facility. Patient reimbursement can also increase enrollment and retention.
Conclusion
Flexible study designs are imperative for assessing product safety during pregnancy. While prospective and retrospective study designs may be sufficient, ambispective designs optimize the benefits of each, while avoiding many of the challenges. Multiple sources of data and multiple perspectives are complementary, and together provide a more holistic view of the pregnant woman and her infant(s)—and the journey through pregnancy, delivery, and beyond. For each new product that comes to market, including COVID-19 vaccines, regulators must decide whether further post-marketing research will be required to assess product safety in pregnancy, and the studies that are designed to meet this objective must be tailored to the characteristics of the product and the patients who are using it.
References
- US Food and Drug Administration. Postapproval Pregnancy Safety Studies Guidance for Industry. May 2019. Available at: https://www.fda.gov/media/124746/download. Accessed April 23, 2021.
- Simeone JC, Nordstrom B. Trends in Post-approval Pregnancy Safety Studies Required by The United States Food and Drug Administration, 2015-2019. Poster presentation at the 36th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (Virtual): September, 2020.
- Lokken EM, Huebner EM, Taylor CG, et al. Disease Severity, Pregnancy Outcomes, and Maternal Deaths Among Pregnant Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Washington State. Am J Obstet Gynecol. 2021 Jan 27;S0002-9378(21)00033-8. doi: 10.1016/j.ajog.2020.12.1221.
- The American College of Obstetricians and Gynecologists. Practice Advisory: Vaccinating Pregnancy and Lactating Patients Against COVID-19. December 2020. Available at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19#:~:text=ACOG%20recommends%20that%20COVID%2D19,similar%20to%20non%2Dlactating%20individuals. Accessed April 23, 2021.