SPRING 2021, THE EVIDENCE FORUM, WHITE PAPER
 Thomas Laurent, PhD Clinical Researcher Clinical Study Support |  Sophie Graham, MSc Research Associate Real-World Evidence Evidera, a PPD business |  Takahiro Hirano, PhD Clinical Researcher Clinical Study Support |  Jason Simeone, PhD Senior Research Scientist and Director of US Database Analytics Real-World Evidence Evidera, a PPD business |
 Ryozo Wakabayashi, PhD Clinical Researcher Clinical Study Support |  Robert Phillips, MSc Researcher and Medical Writer Clinical Study Support |  Tatsuya Isomura, PhD, MSc Founder/CEO Clinical Study Support |
Valuable information for real-world evidence generation can be gleaned from healthcare databases, and the number of databases available continues to expand. Knowing the right data to answer the right question is critical in effective study design. While much is known about use and access of data through Europe and North America, the expanding interest in research in Asia-Pacific presents a new challenge in understanding the uses and challenges of new databases. Japan’s health system and corresponding healthcare databases provide a unique challenge. This article focuses on outlining the Japanese healthcare system, its available real-world databases, and insights into their effective use.
Overview of the Japanese Healthcare System
Japan has universal healthcare coverage for citizens via social health insurance. There are three sub-systems: National Health Insurance (for the self-employed), Employee Health Insurance (for employees), and the Special Scheme for the Aged (for individuals age 75 and older). The insurance systems cover most medical services, in most cases paying 70% of the cost of covered care, with the remaining costs borne by the insured. In some cases, elderly costs are covered at a higher percentage, up to 90%. In all cases, however, the insured pays out of pocket for over-the-counter drugs, normal pregnancy and delivery care, vaccines, and “lifestyle” treatments such as cosmetic surgeries.
Because Japanese employees tend to stay with the same employer for many years, Japan has robust healthcare data compared to nations where employees change jobs or health insurers more often. Annual check-ups are provided by employers at no cost to employees and include blood work, a chest X-ray, height and weight measurements, and vision, hearing, urine, blood pressure, and obesity tests. There is also the option to pay for an annual “Ningen Dock,” a day-long or overnight stay in a hospital for a full health work-up, including an endoscopy, cancer screenings, X-rays, and other tests. Generally, Japanese patients do not have a primary care physician and referrals are not required to see a specialist; however, specialist visits are more expensive without a referral. Patients must consult a doctor for each prescription refill and new prescriptions are often for only two weeks at a time. Prescriptions for long-term or chronic conditions may be given for up to a year, excluding narcotics.
Since insurance claims are submitted by patients and healthcare facilities monthly rather than for each encounter, researchers can see which claims were submitted when but not necessarily the order in which care was given. Japan primarily uses the fee-for-service system; however, a diagnosis procedure combination (DPC) payment system unique to Japan was introduced in 2003 to improve healthcare standards and transparency, and overall institutional performance. Inpatient claims rely on the DPC payment system that groups patients according to diagnosis categories. Inpatient DPC hospitals charge a flat rate, which is calculated by multiplying the rate by the length of the stay, plus additional costs for surgeries or other procedures. Outpatient care is fee-for-service.
Available Real-World Databases
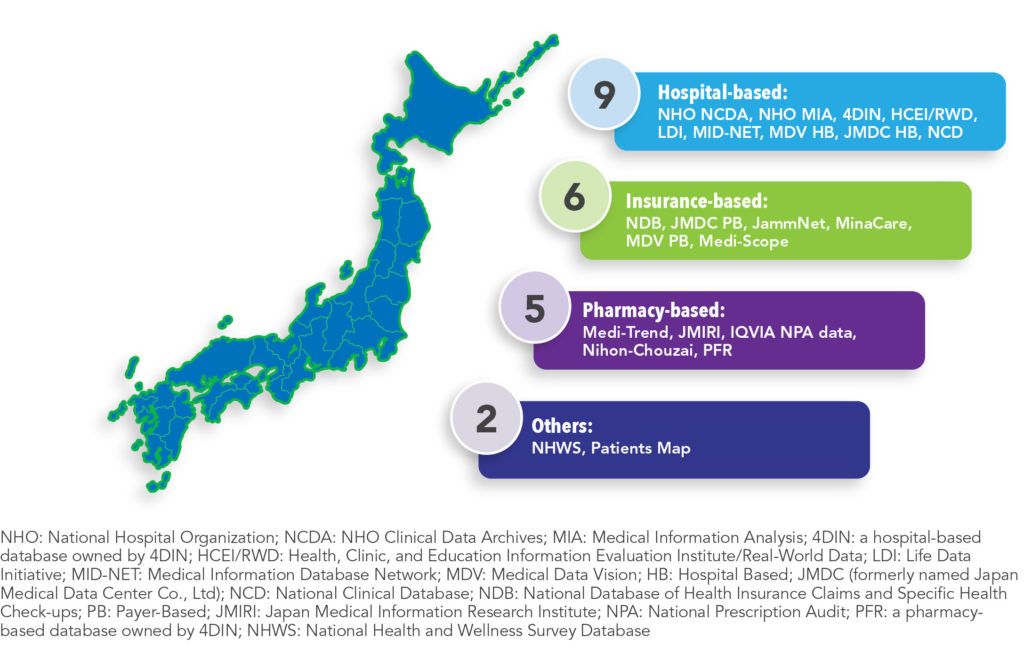
There are 22 databases in Japan that are regularly used in pharmacoepidemiology research (See Figure 1). These data can be classified as either hospital-based (41%), insurance-based (27%), pharmacy-based (23%), or other sources, such as surveys (9%). Eighty-two percent of these data include information on outpatient visits, with 64% including information on medications dispensed in the outpatient setting. Sixty-four percent of the databases include inpatient stay data, with 59% including information on medications dispensed in-hospital. Most databases (64%) record diagnoses using the International Classification of Diseases, Tenth Revision (ICD-10) system, which has been used in Japan since 1995. Nearly half (41%) of the databases indicate whether a laboratory test was ordered, but only 36% record test results.
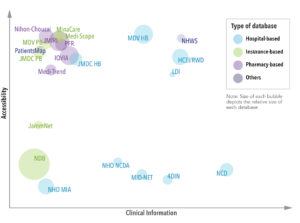
Pharmaceutical companies have access to most of the healthcare databases in Japan. Those most easily accessed (i.e., direct access to the data through a license or one-off payment) include: HCEI/RWD, LDI, MDV (hospital and payer-based), JMDC (hospital and payer-based), Minacare, Medi-Scope, Medi-Trend, JMIRI, IQVIA, Nihon-Chouzai, PFR, NHWS, and PatientsMap. However, some databases, such as JammNet, are only available through indirect access, while others are only accessible to licensed or academic researchers. The databases with the most clinical information (e.g., laboratory, genetic, diagnostics and physiological test results) include NHO NCDA, 4DIN, HCEI/RWD, LDI, MID-NET, MDV, NCD, and NHWS.
The number of people included in each database varies (See Figure 2). The NDB has data on 120 million people, nearly the entire Japanese population. Other databases with significantly large population coverage include the JMIRI (39 million), MDV (33 million), IQVIA (33 million), and HCEI/RWD (21 million).
Considerations and Recommendations
Japan has several robust healthcare databases that are proving to be valuable in real-world evidence generation. However, there are also some unique challenges in using this data. Here are some key considerations and recommendations in using Japanese data.
Data Access
Many databases have limitations on their availability for outside researchers. For example, NDB, NCD, 4DIN, MID-NET, and the NHO datasets are not directly accessible to pharmaceutical companies. There are also logistical restrictions. For example, some provide the data on a flash drive and there are restrictions on shipping outside Japan.
Recommendation: Collaborate with local researchers who can access these data.
Language Barriers
Relevant clinical documentation is often in Japanese. Specifically, treatment guidelines for rare diseases, drug package inserts, data dictionaries, diagnosis, and receipt codes dictionaries are often not available in other languages. Access to this information is critical for appropriately reflecting clinical practice patterns in Japan during the design and data interpretation phases of a study.
Recommendation: Work with a Japanese translator with knowledge of the database being used in the study.
Longitudinality of the Data
It can be difficult to track patients in most Japanese hospital-based databases because visits to other institutions within the data network cannot be linked as each facility uses a unique identifier. In an insurance-based claims database, patients retain the same identifier if they maintain the same insurance policy.
Recommendation: When designing a study, if it is important to adjust for confounding variables at index, or if continuous follow-up of patients is required, then the use of insurance-based claims is recommended.
Data Coverage
Data from insurance-based claims are limited to only working-age patients and their dependents. However, hospital-based databases have their own limitations. For example, large hospitals and hospitals that admit patients with more severe conditions, such as DPC-designated hospitals, may be overrepresented.
Recommendation: Consider the target population in a study before selecting a data source. If the study primarily focuses on the elderly population, use a hospital-based database.
Data Source Quality
For some databases, especially hospital-based claims databases, demographic information such as weight, height, and other variables like smoking status may be missing. Discharge summaries at DPC-designated hospitals may lack information that is not relevant for reimbursement purposes, even if the variable exists in the database. In addition, laboratory test results are available in hospital-based databases, such as MDV, but the set of institutions providing this information might be limited.
Recommendation: Restricting the analysis to patients with available data should be carefully considered as this could strongly impact the generalizability of the analysis.
Conclusion
Several Japanese databases, such as MDV and JMDC, are available to researchers and are frequently used to conduct real-world studies. A careful assessment of each database’s strengths and limitations is highly recommended before selecting a database for use in a study. Additionally, the structure of the Japanese healthcare system and the way that care is delivered to patients is unique compared to other countries in North America and Europe. It is particularly important to understand these factors or to collaborate with local researchers, as they may influence both the study design and interpretation of evidence derived from real-world studies.