FALL 2020, THE EVIDENCE FORUM, WHITE PAPER
 Jennifer Boss, MSc Senior Market Access Writer Evidence Synthesis, Modeling & Communication Evidera, a PPD business |  Jessica Griffiths, MEnt Senior Consultant Value & Development Consulting Evidera, a PPD business |  William Laughlin, PhD Senior Business Analyst Value & Development Consulting Evidera, a PPD business |  Christian Vanoni, MScEng, MIHMEP Associate Consultant Value & Development Consulting Evidera, a PPD business |
 Almudena Olid Gonzalez, MA Consultant Value & Development Consulting Evidera, a PPD business |  Kacey Rawson, MSc Associate Scientific Director Evidence Synthesis, Modeling & Communication Evidera, a PPD business |  Matthew Bending, PhD, MSc Executive Director of HTA Strategy & UK Practice Lead Value & Development Consulting Evidera, a PPD business |
ISA and the Value of Multiple Stakeholder Engagements
Multi-stakeholder involvement with patients, regulators, and health technology assessment (HTA) bodies is fundamental in the development of evidence generation plans for the success of new technologies.1 Medical treatment developers can seek to optimize their plans via Integrated Scientific Advice (ISA), through which regulators, HTA bodies, and payers (see Figure 1) are able to provide constructive feedback, enabling developers to create a robust evidence package that is relevant to all stakeholders, including patients, clinicians, regulators, HTA bodies, and payers, paving the way for timely access.1
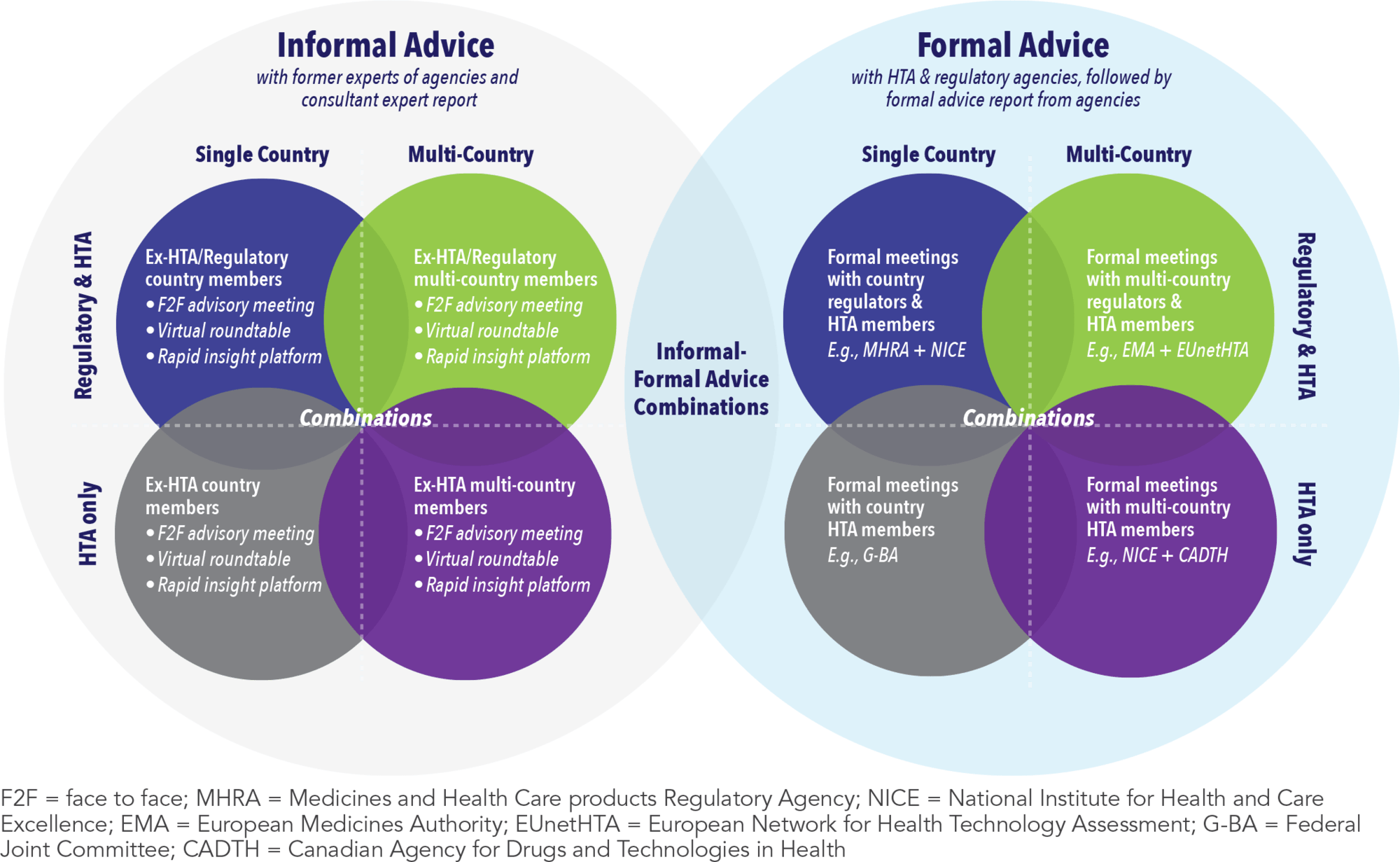
Since the establishment of the first HTA early advice procedure in 2009, the number of options available to treatment developers has dramatically increased. In addition to the options for ISA offered by national regulatory agencies and HTA bodies, several multinational programs have emerged such as the European Network for Health Technology Assessment (EUnetHTA) Early Dialogues and the National Institute for Health and Care Excellence (NICE)-Canadian Agency for Drugs and Technologies in Health (CADTH) parallel advice. These formal, national and multinational interactions can be further supplemented through informal advice with former members of the agencies via advisory meetings and roundtables, among other engagements. Different combinations of formal and informal engagements can also be sought to maximise the value of these interactions while at the same time meeting key internal objectives, deadlines, and resource requirements (See Figure 2).
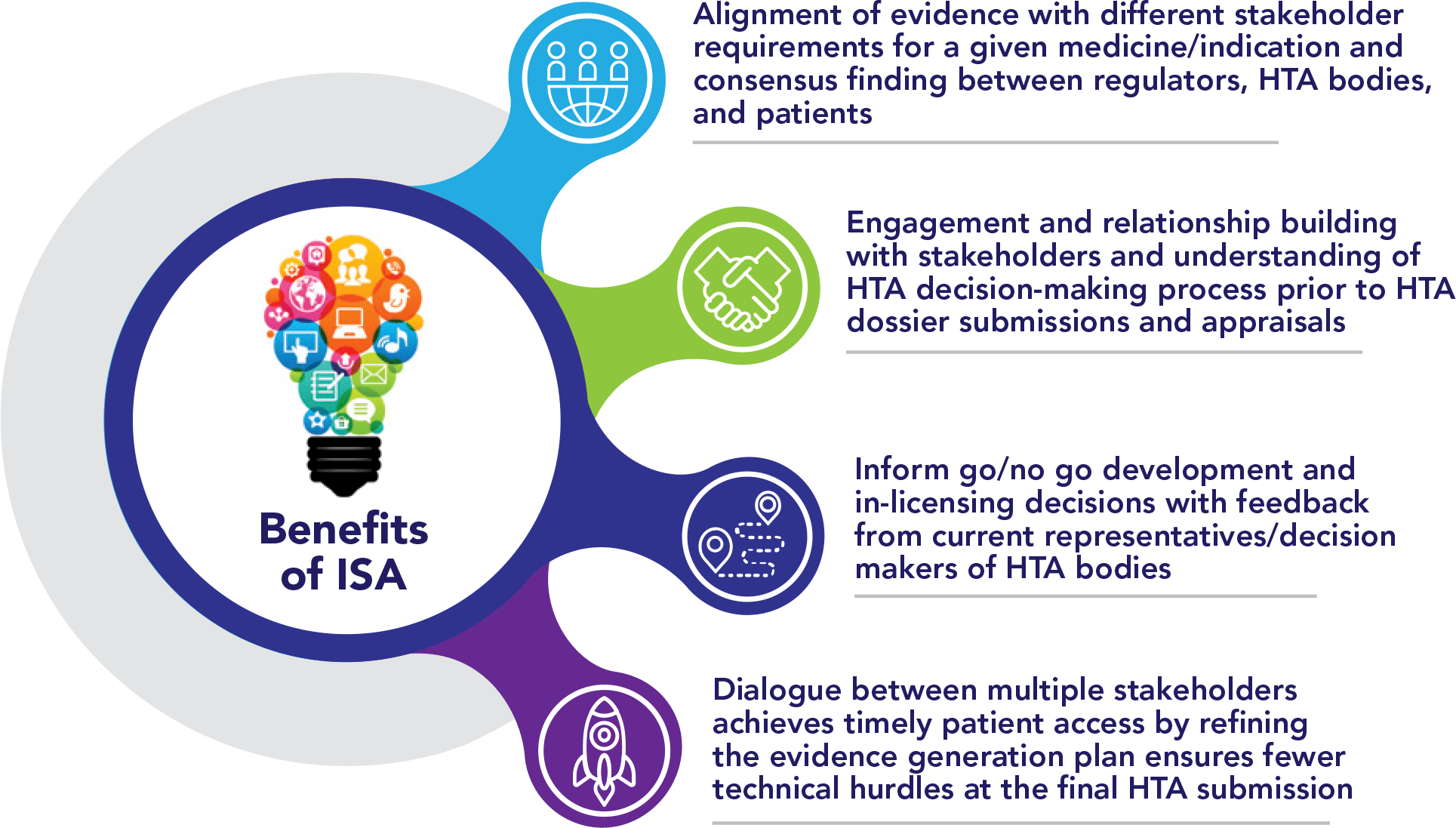
Despite being a relatively recently established procedure, there is emerging evidence of the benefits of ISA for developers.2 Agreement between stakeholders on evidence generation topics discussed during the scientific advice procedure has generally been high, especially among HTA bodies where a consistently high level of agreement is observed.2, 3 While the views of European Union (EU) HTA bodies and regulators can vary, choice of treatment comparator is the only domain where a meaningful variance in agreement has been found.2, 3 Treatment developer compliance with provided advice is higher for regulatory advice than for HTA advice, especially when the stakeholders have limited alignment on their advice.2, 3 The benefits of ISA to developers fall into four main streams (See Figure 3)1,2:
De-risking
Companies can obtain external validation of their clinical development plan. This is particularly useful for medicines with transformative potential, are first-in-class, there is complexity or uncertainty in the approach to clinical development, and where there is a lack of clinical or HTA guidelines.
Engagement
The process provides an opportunity for early engagement across market access stakeholders; for example, incorporating a patient representative can provide crucial insights into the unmet need and patient burden associated with a given indication, which may challenge pre-conceived notions around the need for new therapeutic innovation.
Alignment
The process requires the cross-functional involvement of clinical, biostatistics, regulatory, market access, health economics, and outcomes research within a company. This early cross-functional engagement may lead to improved internal alignment during the preparation process, exposure of functions to the HTA decision making process, as well as educating different functions on the HTA appraisal process and market access, leading to greater internal harmony on the future development of other treatments.
Timely patient access
The opportunity to seek scientific advice with multiple stakeholders and refine the evidence generation plan and launch strategy in response to feedback ensures fewer technical hurdles at the final HTA submission. There is emerging evidence of the positive impact of obtaining early ISA on the subsequent achievement of positive HTA recommendations.1-4
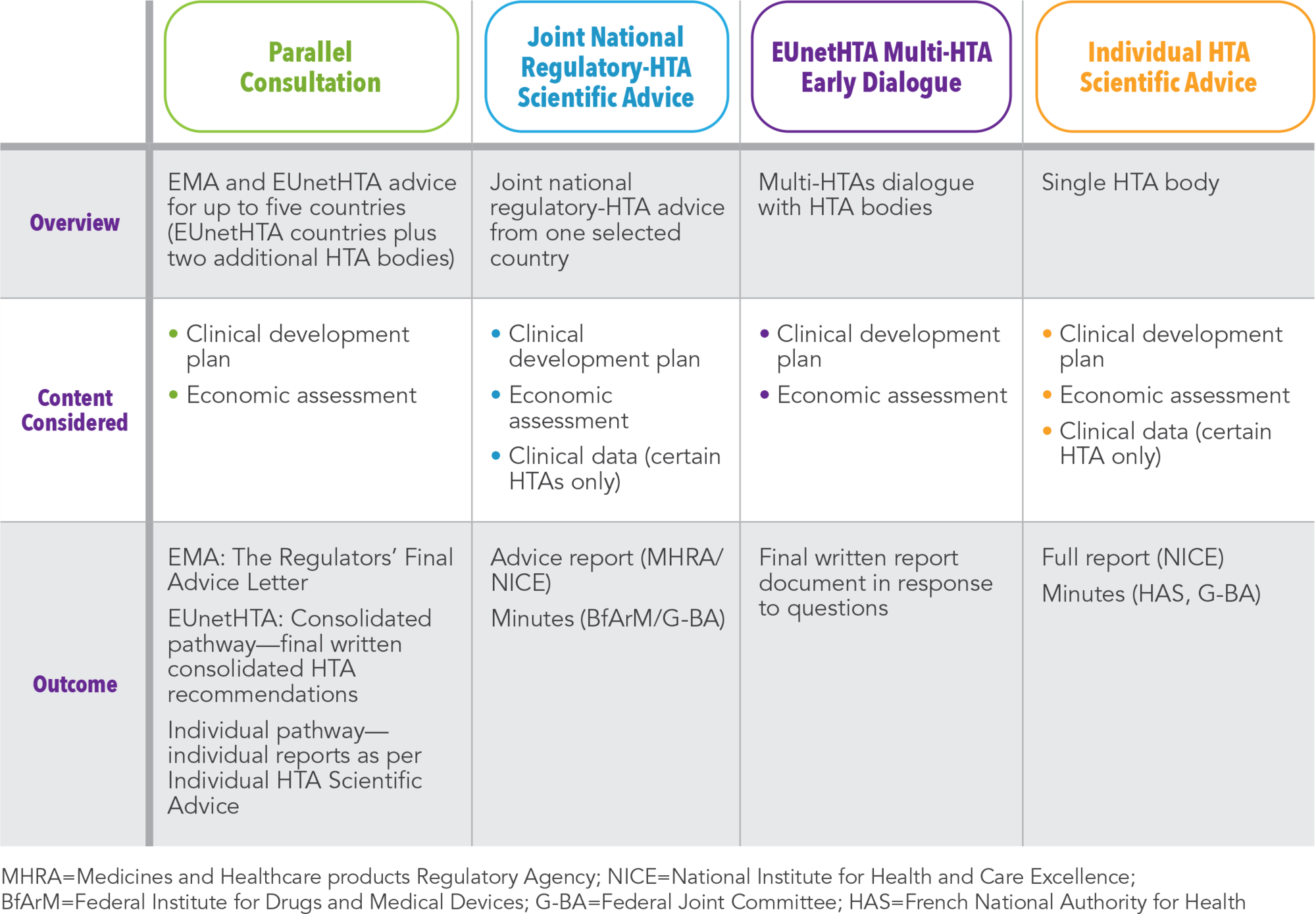
While early engagement with regulatory bodies for advice on clinical and non-clinical development plans to demonstrate the safety and efficacy data required for marketing authorization is well established, the involvement of HTA bodies in scientific advice is relatively recent. The first HTA advice procedure was only established in 2009 compared with the much earlier establishment of regulatory advice in 1995. HTA bodies provide advice on the evidence requirements to demonstrate relative effectiveness and economic value of a new product in clinical practice compared with the current standard of care (See Table 1). HTA bodies can also provide direction on relevant comparators, outcomes of interest, evidence quality, and relevance of routine clinical practice. As for HTA appraisals, the patient perspective is increasingly being incorporated at the advice stage.
The ISA Framework
With the growing availability of ISA there is a need to describe and classify ISA procedures to support comparisons and identify the value of the differing programs, similar to the HTA framework developed by Hutton et al.5,6 Evidera has developed an initial and detailed framework to compare formal ISA procedures, due to be presented at ISPOR Europe 2020 in November. A key aim of this framework is to provide details on the different services that are offered to companies seeking advice in a standardised format, thus making it possible to compare across ISA offerings.
Hutton et al.5,6 developed an analytical framework to describe and classify the requirements used to justify the reimbursement of pharmaceuticals by health systems. This comprehensive analytical framework provides a landmark to collect information on characteristics of HTA systems in a more systematic way. This methodology enables us to move toward a consensus on the key characteristics of HTA bodies, thereby facilitating cross-country comparison.
Using the Hutton HTA framework as our base, we performed a desk review of websites, public domain sources, and interviews with agency representatives to inform an internal workshop; the result was the creation of an analytical framework for ISA procedures that describes and classifies procedures based on identifiable categories for comparison. Available ISA procedures were compared using the framework and current trends and developments identified. We previously described the impact of COVID-19 on ISA procedures in an earlier white paper titled “Integrated Scientific Advice during the COVID-19 Pandemic: A Status Update on Key Programs in North America and Europe,” allowing us to also include the impact of COVID-19 into our framework. All programs had or continue to have at least some procedural modifications, with EUnetHTA, Canadian, and Italian procedures being subject to temporary suspensions. Other modifications included:
- Shift to online meetings and option for written advice only
- Capacity restrictions and prioritization of therapeutically critical technologies
- Accelerated procedures for therapeutically critical indications
- Further detail on company evidence generation plans required, e.g., real-world evidence (RWE) and patient-reported outcomes (PRO) sections
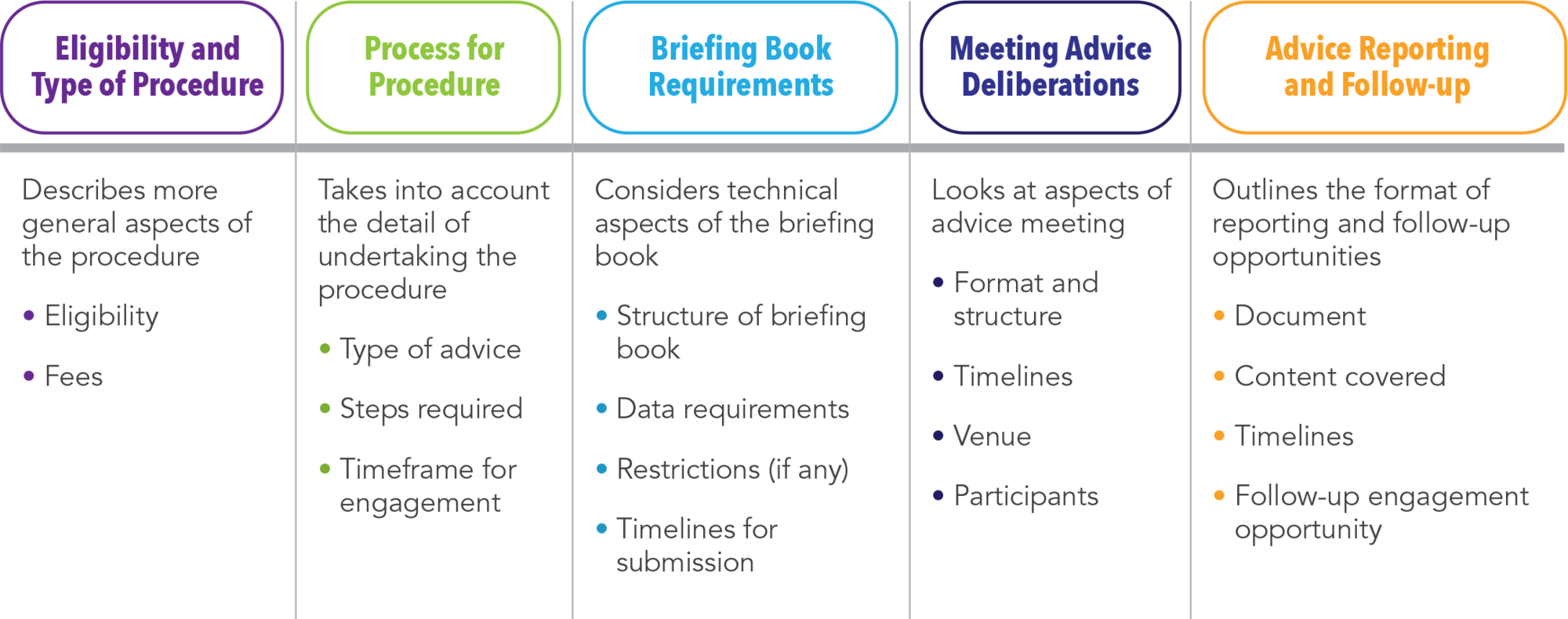
Based on our investigations, we developed an ISA framework that classifies the characteristics of ISA procedures into five distinct categories (See Table 2).
The proposed framework was then validated by analysing various ISA offerings within individual countries and globally. The results from this analysis will be presented at ISPOR Europe 2020. In brief, general trends included:
- Increased demand for ISA and resulting agency capacity issues
- Impact of COVID-19 leading to changes in existing procedure processes and development of new offerings, including:
- Prioritization of COVID-19-related or therapeutically critical technologies
- Acceleration of processes (e.g., new fast-track options)
- Change of meetings to virtual format
- More detailed submission requirements, such as companies needing to submit patient-reported outcomes and post-launch evidence plans
While more research is ongoing, the proposed framework has enabled our team to identify key risks arising from the COVID-19 situation as well as changes and trends for some of the key ISA procedures globally. Given the differences between each procedure and the complexities of the engagements, it is important for companies to assess all options before requesting ISA, with a keen understanding of their objectives, the expected value to be gained from different engagements, and any potential associated risks. By providing the basis for comparative assessment between ISA procedures, this framework is aimed at helping to navigate the diversity of these procedures and identify the most appropriate approach to ISA based on a company’s asset and strategy.
References
- Udechuku A, Bending M. The Value of Health Technology Assessment Scientific Advice. Regulatory Focus. 2017 August 23.
- Ng T, Ziomek J, Delaitre-Bonnin C, Bending MW. A Review of the Impact of Integrated Scientific Advice for the Optimisation of Evidence Generation for HTA Appraisals. Value Health. 2018. 21: S197.
- Ziomek J, Franklin TN, Bending MW, Delaitre-Bonnin C. (2018) A Review of the Impact of Integrated Scientific Advice for the Optimisation of Evidence Generation for HTA appraisals (Poster). ICONplc.com
- Morton R, Bending M, Latour S. The Critical Role of Medical Affairs in Value Story Development and Integrated Scientific Advice. Presented at 2018 MAPS EMEA Annual meeting.
- Hutton J, McGrath C, Frybourg JM, Tremblay M, Bramley-Harker E, Henshall C. Framework for Describing and Classifying Decision-Making Systems Using Technology Assessment to Determine the Reimbursement of Health Technologies (Fourth Hurdle Systems). Int J Technol Assess Health Care. Winter 2006;22(1):10-8. doi: 10.1017/s0266462306050781.
- Beletsi A, Koutrafouri V, Karampli E, Pavi E. Comparing Use of Health Technology Assessment in Pharmaceutical Policy among Earlier and More Recent Adopters in the European Union. Value Health Reg Issues. 2018 Sep; 16:81-91. doi: 10.1016/j.vhri.2018.08.002. Epub 2018 Oct 11.
For more information, please contact
[email protected], [email protected], [email protected], [email protected], [email protected], [email protected], or [email protected]