FALL 2020, THE EVIDENCE FORUM, WHITE PAPER
 Jennifer Boss, MSc Senior Market Access Writer Evidence Synthesis, Modeling & Communication Evidera, a PPD business |  Matthew Bending, PhD Executive Director of HTA Strategy & UK Practice Lead Value & Development Consulting Evidera, a PPD business |  Kacey Rawson, MSc Associate Scientific Director Evidence Synthesis, Modeling & Communication Evidera, a PPD business |
The global COVID-19 pandemic has infiltrated every aspect of daily life with long-lasting effects expected across all sectors, including health technology assessment (HTA) and the market access of pharmaceuticals. Healthcare treatment developers must brace themselves for considerable delays to clinical trials, re-prioritization of pipeline products, and a shift change in normal working practices. As a follow-up to our investigation on the impact of COVID-19 on integrated scientific advice procedures (See “Integrated Scientific Advice during the COVID-19 Pandemic: A Status Update on Key Programs in North America and Europe” in the Spring 2020 issue of The Evidence Forum), we now focus on another key area of risk to the commercialization of a new HTA and price negotiations.
HTA Overview
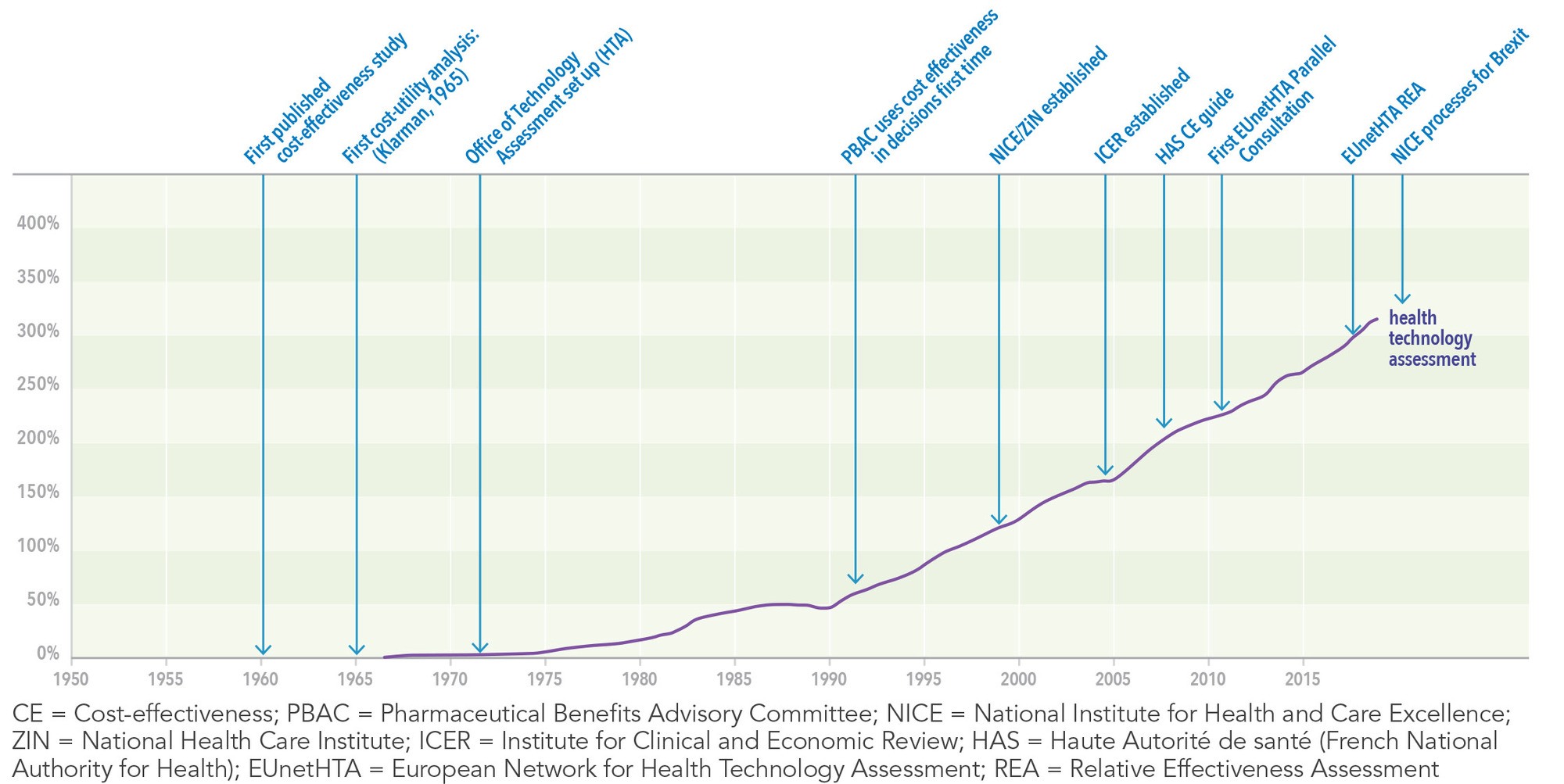
Health technology assessment is a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle.1 The purpose is to inform decision making in order to promote an equitable, efficient, and high-quality health system.1 Distinct from integrated scientific advice which takes place at an earlier stage in the product lifecycle during evidence generation planning (See “An Initial Framework to Describe and Classify Integrated Scientific Advice Procedures: Trends and Developments” in the Fall 2020 issue of The Evidence Forum), HTA is an evaluative approach that assesses the impact on society of health technologies and informs decision making regarding the reimbursement of drugs and other health technologies.2 Since its creation in the 1990’s, HTA has become widely implemented across health systems globally and is now a key stage for successful market access.2 As one indicator of its use, the term “health technology assessment” was entered into Google Ngram Viewer, which displays a graph showing how that phrase has occurred in a corpus of books (e.g., British English, English Fiction, French) over a selected period of years. The frequency with which the phrase is used in books highlights how quickly the terminology has become adopted (See Figure 1). Indeed, justification of reimbursement is now commonly referred to as the fourth hurdle to obtaining a product license after demonstration of product quality, efficacy, and safety.
Throughout 2020, the COVID-19 pandemic has resulted in changes to the status of HTA as institutions reallocate resources to respond to outbreak-related requests and healthcare practitioners provide care to those affected by the pandemic. While there are resulting delays and disruptions to various HTA programs across countries, there are still options available for sponsors seeking assessment and appraisal.
Status of HTA During the Pandemic
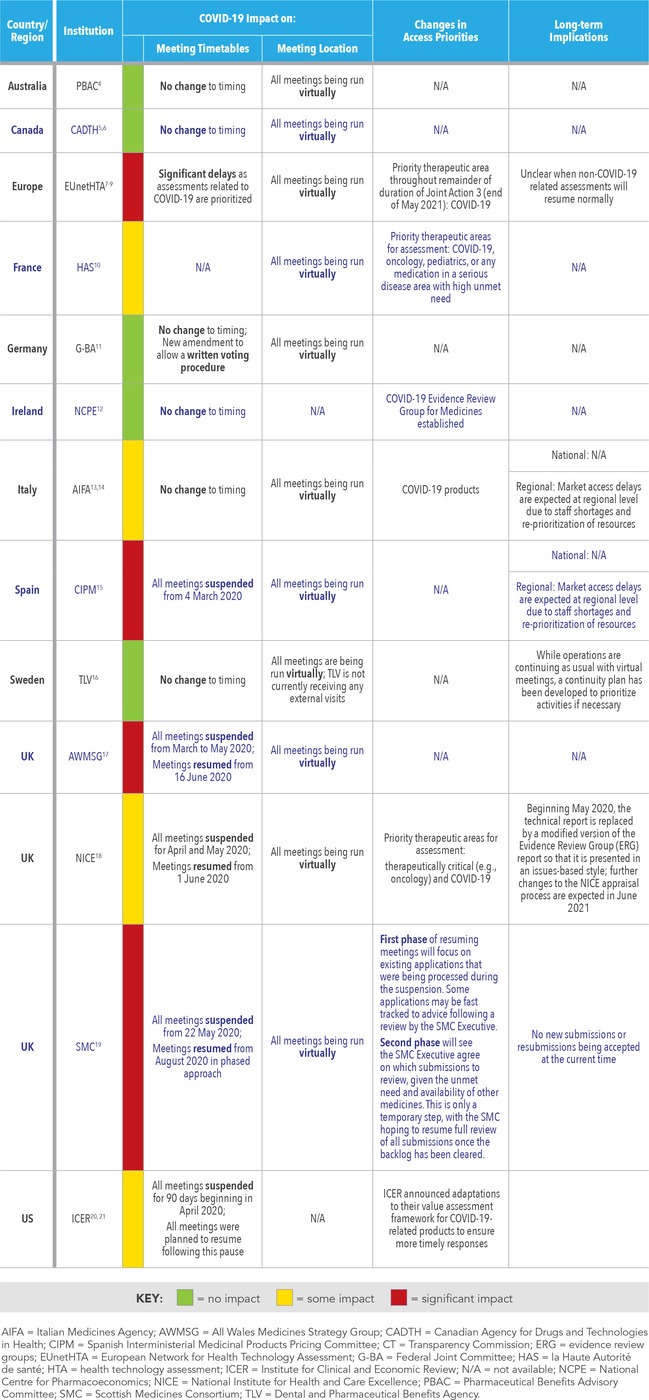
A summary of the status of key HTA programs in North America, Europe, and the Asia-Pacific region is presented in Table 1. COVID-19 has had a varying impact on market access across the HTA programs in terms of changes to the format and timeframes of assessment meetings (whereby interactions between the HTA body, sponsor, and Evidence Review Group are held), and prioritization of technologies by the institutions.
Format
The most widespread change is the switch of meeting format from in-person to virtual which has been implemented across each program by all institutions.
Timeframe
In terms of timeframes, some institutions are conducting their HTA programs as normal with minimal or no impact on assessment timeframes, including the Pharmaceutical Benefits Advisory Committee (PBAC; Australia), Canadian Agency for Drugs and Technologies in Health (CADTH; Canada), Federal Joint Committee (G-BA; Germany), Italian Medicines Agency (AIFA; Italy), National Centre for Pharmacoeconomics (NCPE; Ireland), and Dental and Pharmaceutical Benefits Agency (TLV; Sweden). Other institutions have temporarily suspended their HTA programs during the pandemic, including the Spanish Interministerial Medicinal Products Pricing Committee (CIPM; Spain). Although the HAS in France did not suspend meetings, priority therapeutic areas for assessment have been identified (COVID-19, oncology, pediatrics, or any medication in a serious disease with high unmet need) and products which do not fall into these categories are expected to be delayed.
In the United Kingdom, the All Wales Medicines Strategy Group (AWMSG; Wales), National Institute for Health and Care Excellence (NICE; England), and Scottish Medicines Consortium (SMC; Scotland) had temporarily suspended all appraisal meetings for varying lengths of time during the first and second quarters of 2020; however, all three institutions have now resumed their HTA programs which are running with delays (note that the SMC is not currently accepting new submissions or resubmissions). Similarly, the Institute for Clinical and Economic Review (ICER) in the United States (US) also temporarily suspended assessment meetings, but activities have now resumed with a 90-day delay in assessments as of April 2020.
Prioritization of Specific Therapy Areas
Multiple institutions have announced prioritization of certain therapeutic areas for assessment, including products for COVID-19 (including HAS, AIFA, NICE, EUnetHTA, and ICER) or therapeutically critical products for oncology, pediatrics, or diseases with a high unmet need (HAS and NICE).
Short-term Implications
The most immediate challenge facing HTA agencies as they resume normal operations is to clear the backlog of appraisals. For HTA programs such as those at NICE and SMC which have resumed meetings following temporary suspension, delays of at least six months are expected through 2021 (especially for products in non-prioritized therapeutic areas). Although some HTA agencies are trying to help with backlog through the prioritization of disease areas with high unmet need, the criteria for prioritization is not always transparent and this approach is not consistent between the agencies assessed. As a result, considerable variations in delay timeframes across different indications and markets is to be expected.
Recommendation: The exact impact of the predicted delays on the launch timelines of specific products remains to be seen; however, pharmaceutical companies should be reassessing country launch waves, cross-country launch sequencing, and competitor launch forecasting for all late stage products in order to identify new opportunities and prepare for emerging challenges.
Mid-term Implications
All countries assessed have now adopted virtual meetings with face-to-face interactions postponed for the foreseeable future. Therefore, market access teams must adjust their preparations from a face-to-face to a virtual platform. In a virtual setting, it becomes more difficult to communicate within your own team, react to non-verbal cues, and to generate a level of familiarity among attendees. In addition, those attending the meeting will be more reliant on pre-submitted written materials.
Recommendation: Clear and concise value communication across all written deliverables should be prioritized alongside virtual negotiation preparedness training with affiliate teams. Some best practices include:
- Restart computer at least 30 minutes before the meeting with HTA and keep all unnecessary applications closed (including email, as it can be distracting)
- Test video and audio the day before (e.g., Zoom, the platform used by NICE, allows the ability to do a test meeting to check audio and video)
- Keep a separate chat open outside of the HTA meeting for within-team communication
- Ensure microphone is muted when not speaking
- Be aware of one’s location and surroundings, ensuring no distractions, room lighting is adequate, etc.
- Dress as you would for a face-to-face meeting
The development of virtual negotiation playbooks, negotiation guidelines, and mock negotiations will enable affiliate teams to suitably prepare for this new setting.
Long-term Implications
The COVID-19 pandemic has put healthcare systems around the world under considerable pressure, and a long-term shift in priorities is predicted across all countries. In addition, the considerable financial cost of dealing with the pandemic will mean healthcare budgets will be even more constrained. This is anticipated to create knock-on effects for pharmaceutical companies; greater difficulty in proving the value of new medications for market access approval, tougher pricing negotiations, and stricter volume restrictions are expected. A lower willingness to pay due to COVID-19 will likely lead to greater evidence requirements in submission dossiers during a time when conducting additional clinical trials is difficult. It is also possible changes to formal or informal thresholds of budget impact and/or cost-effectiveness or international reference pricing approaches may also be forthcoming. NICE, for example, has already announced changes to the HTA appraisal process based on learnings from changes to working practices during the pandemic.3
Recommendation: An end date to the prioritization of certain disease areas was not provided by any of the HTA agencies assessed for this article. Therefore, one can only speculate on what this means for the future of prioritization, but it is possible that agencies will move to a new model where products meeting a defined set of criteria no longer need formal appraisal as a way to free up resources long term. Early and continued engagement with HTA bodies will provide useful insights into this evolving payer landscape. While early stage interactions with HTA and regulatory bodies in the form of integrated scientific advice is emerging to be very beneficial, it may be invaluable during this period of growing uncertainty. As we published previously, many agencies are still offering scientific advice services (albeit remotely) helping companies to understand stakeholder needs and refine their evidence package. In addition, it will be important for pharmaceutical companies to be open to alternative and innovative access arrangements, and aligned customer engagement across the local health ecosystem will be essential to successfully negotiate all stakeholders and decision makers. This will require complex analysis, planning, and engagement at a local level, valuing the contribution of each role.
References
- O’Rourke B, Oortwijn W, Schuller T, International Joint Task Group. The New Definition of Health Technology Assessment: A Milestone in International Collaboration. Int J Technol Assess Health Care. 2020 Jun;36(3):198-190. doi: 10.1017/S0266462320000215. Epub 2020 May 13.
- Drummond M, Sorenson C. Nasty or Nice? A Perspective on the Use of Health Technology Assessment in the United Kingdom. Value Health. 2009 Jun;12 Suppl;12 Suppl 2:S8-13. doi: 10.1111/j.1524-4733.2009.00552.x.
- National Institute for Health and Care Excellence (NICE). Coronavirus (COVID-19). Available at: https://www.nice.org.uk/covid-19. Accessed September 7, 2020.
- Australian Government Department of Health. The Pharmaceutical Benefits Scheme. Available at: https://www.pbs.gov.au/info/news. Accessed September 7, 2020.
- CADTH. COVID-19 Update. Available at: https://www.cadth.ca/about-cadth/what-we-do/products-services/cdr/covid-19-update. Accessed September 7, 2020.
- CADTH. CADTH Pharmaceutical Reviews Update – Issue 15. Available at: https://www.cadth.ca/cadth-pharmaceutical-reviews-update-issue-15. Accessed September 7, 2020.
- European Network for Health Technology Assessment (EUnetHTA). COVID-19 Response. Available at: https://eunethta.eu/services/covid-19/. Accessed on September 7, 2020.
- European Network for Health Technology Assessment (EUnetHTA). Statement from the EUnetHTA Secretariat on COVID-19. Available at: https://eunethta.eu/statement-from-the-eunethta-secretariat-on-covid-19/. Accessed September 7, 2020.
- EUnetHTA. EUnetHTA Response to COVID-19. Available at: https://eunethta.eu/eunethta-response-to-covid-19/. Accessed September 7, 2020.
- Haute Autorité de Santé. Commissions Prioritize Health Products to be Evaluated. Available at: https://www.has-sante.fr/jcms/p_3167555/fr/les-commissions-priorisent-lesproduits-desante-a-evaluer. Accessed September 7, 2020.
- Gemeinsamer Bundesausschuss (G-BA). Public Meetings of the G-BA. Available at: https://www.g-ba.de/service/oeffentliche-sitzungen/. Accessed September 7, 2020.
- National Centre for Pharmacoeconomics (NCPE Ireland). COVID-19. Available at: http://www.ncpe.ie/news/covid-19/. Accessed September 7, 2020.
- AIFA Italian Pharmaceutical Agency. Ristabilite le ordinarie modalità operative dei Registri di monitoraggio AIFA dopo la fase di sospensione per emergenza epidemiologica da COVID-19. Available at: https://www.aifa.gov.it/web/guest/-/ristabilite-le-ordinarie-modalita-operative-dei-registri-di-monitoraggio-aifa-dopo-la-fase-di-sospensione-per-emergenza-epidemiologica-da-covid-19. Accessed September 16, 2020.
- AIFA Italian Pharmaceutical Agency. Extending the Compilation of AIFA Monitoring Logs. Transitional Measures for Containment and Management of the Epidemiological Emergency from COVID-19. Available at: https://www.aifa.gov.it/-/proroga-della-compilazione-dei-registri-di-monitoraggio-aifa. Accessed September 7, 2020.
- Gobierno de Espana. Comision Interministerial de Precios de Medicamentos y Productos Sanitarios. Available at: https://www.mscbs.gob.es/profesionales/farmacia/CIPMyPS.htm?utm_source=diariofarma&utm_mediumnk. Accessed September 17, 2020.
- The Dental and Pharmaceutical Benefits Agency (TLV). COVID19 and Processing Times. Available at: https://www.tlv.se/om-oss/press/nyheter/arkiv/2020-03-23-covid19-och-handlaggningstider.html. Accessed September 7, 2020.
- All Wales Medicines Strategy Group. COVID-19 Update. Medicines Advice and Medicines Optimization Resources. Available at: http://www.awmsg.org/meetings_awmsg_2020.html. Accessed September 7, 2020.
- National Institute for Health and Care Excellence (NICE). Coronavirus (COVID-19). Available at: https://www.nice.org.uk/covid-19. Accessed September 7, 2020.
- Scottish Medicines Consortium. SMC Response to COVID-19. Available at: https://www.scottishmedicines.org.uk/about-us/latest-updates/smc-response-to-covid-19. Accessed September 17, 2020.
- Institute for Clinical and Economic Review (ICER). ICER Indefinitely Postpones Public Meetings for Sickle Cell Disease and Cystic Fibrosis: Expands Other Assessment Timelines Up to Three Months. Available at: https://icer-review.org/announcements/covid19_hiatus/. Accessed September 7, 2020.
- ICER. Adaptions to the ICER Methods for Evaluation of Therapies for COVID-19. 2020 July 18. Available at: https://icer-review.org/wp-content/uploads/2020/07/Adaptations-to-the-ICER-methods-for-evaluation-related-to-treatments-for-COVID_July18.pdf. Accessed September 17, 2020.
For more information, please contact
[email protected], [email protected], or [email protected].