FALL 2020, THE EVIDENCE FORUM, WHITE PAPER
 Delphine Saragoussi, MD, MScPH Senior Research Scientist and Senior Director Real-World Evidence Evidera, a PPD business |  Alice Rouleau, PharmD, MScPH Research Scientist Real-World Evidence Evidera, a PPD business |  Javier Cid, MD, DrPH, MBA, MSc Senior Research Scientist Real-World Evidence Evidera, a PPD business |
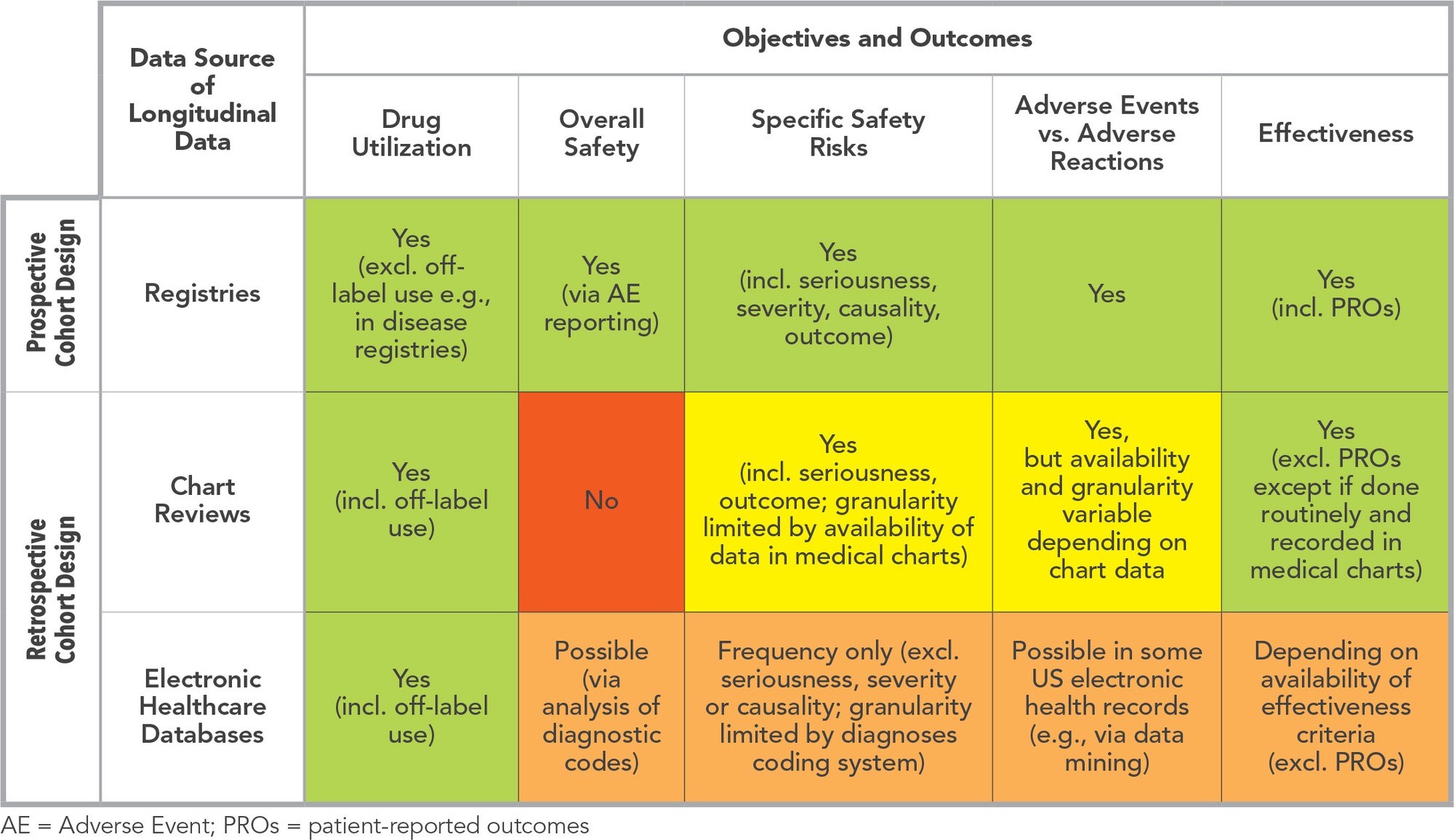
Most often, drug safety studies have a longitudinal cohort study design to describe drug utilization over time and to characterize the risk functions of safety events of interest. Retrospective or historical cohort studies, using retrospective data sources such as medical chart reviews or electronic healthcare databases (e.g., claims or electronic medical records), are often a preferred approach since they have reputedly shorter timelines and lower budgets. Conversely, registries, which are based on a prospective cohort design, might often be viewed as a burdensome approach. However, a post-marketing safety registry can generate an important added value if it is built for the right objectives in the right circumstances.
What is a Registry?
A registry can be defined as “an organised system that uses observational methods to collect uniform data on specified outcomes in a population defined by a particular disease, condition or exposure.”1 Practically, a patient registry is a particular case of a prospective cohort study, which can be open-ended, where the data collection is systematic and usually relies on data arising from routine clinical assessments. Patient registries are not necessarily built for a specific objective but may be built as a framework or data source for sub-studies in the therapeutic area of interest.2,3 This definition corresponds well to “disease registries,” where patients are included if they are diagnosed with a specific disease, with no restriction on how those patients are managed and treated. Disease registries are frequent in rare diseases and have several applications, such as quantifying and characterizing the patient population, evaluating burden of illness, and describing standard of care.
However, registries in the context of post-marketing safety evaluation aim at evaluating the safety of a specific drug and are most of the time “product registries,” where patients are included if they are routinely prescribed a certain drug or group of drugs. Product registries can also be described as prospective cohort studies of patients exposed to one or several drugs.
Why and When to Propose a Product Registry as a Post-Marketing Safety Study?
It may happen that regulatory authorities in one or several geographies request a registry as a post-marketing safety study requirement. A preferred option is always for a market authorization holder to be proactive and take the first step in proposing a safety study to the authorities when planning risk management activities. The choice of the study design should ideally be based on:
- Assessment of the safety risks that will need to be studied in the post-marketing period based on best knowledge of the drug’s safety profile
- Translation of the safety risks into research questions
- Assessment of potential existing data sources to address the research questions
- Appreciation of study design possibilities to best address the research questions
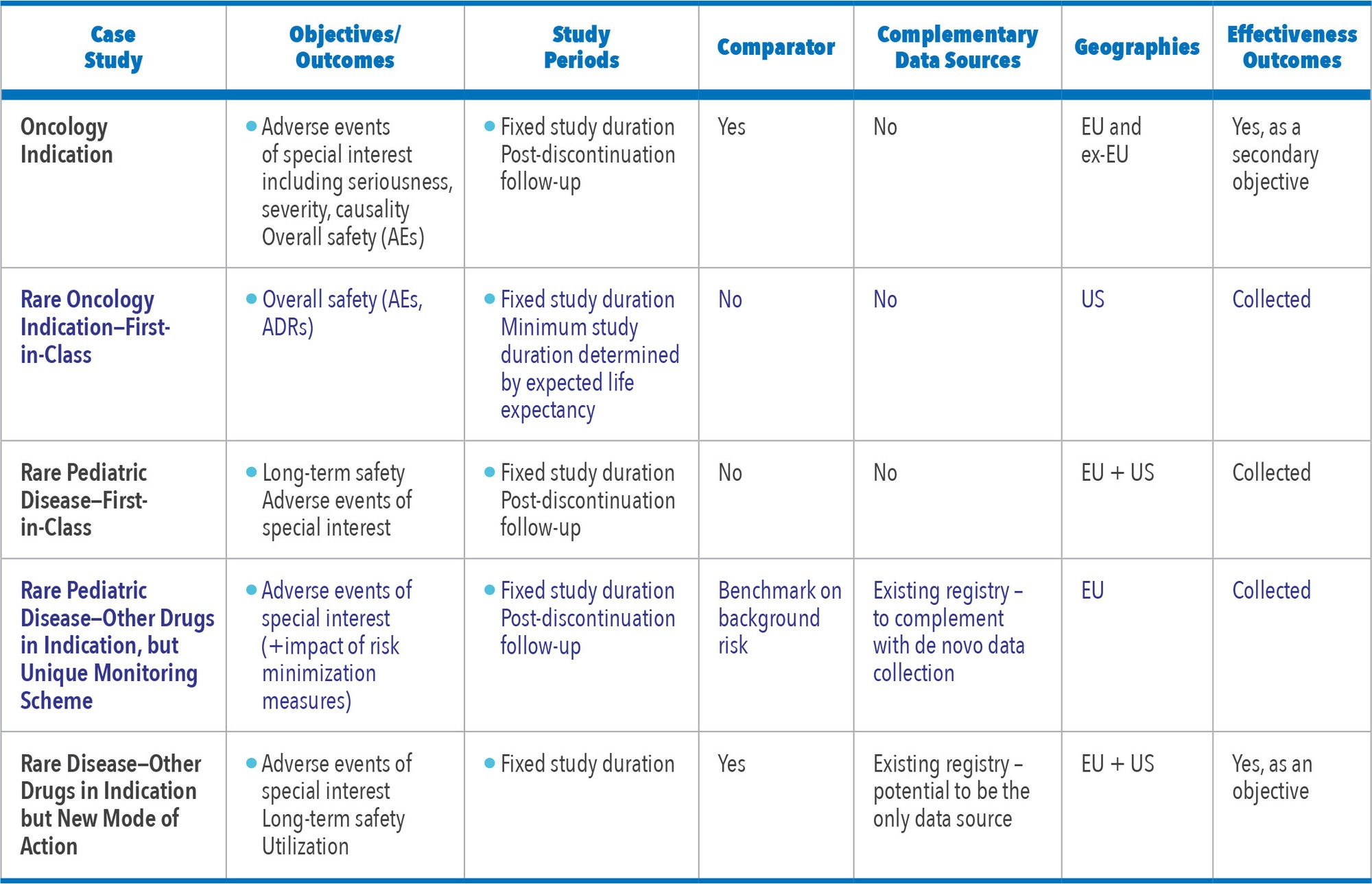
Figure 1 shows the different possible study objectives by type of approach.
A registry can be proposed based on the following cumulative criteria:
- Study objectives calling for prospective longitudinal data collection
- Longitudinal and especially long-term patient follow-up
- Detailed description of safety data (e.g., serious adverse events, suspected adverse drug reactions, suspected unexpected adverse drug reactions)
- High granularity of safety data (e.g., severity, outcomes)
- Detailed clinical data required
- Patient-reported outcomes are of interest
- Circumstances calling for a targeted prospective primary data collection
- Rare disease
- Disease not identifiable in an electronic healthcare database
- Absence of available or appropriate electronic healthcare database
- Need to monitor safety in “real-time” and regularly report to authorities (e.g., interim analyses, yearly updates to regulatory authorities)
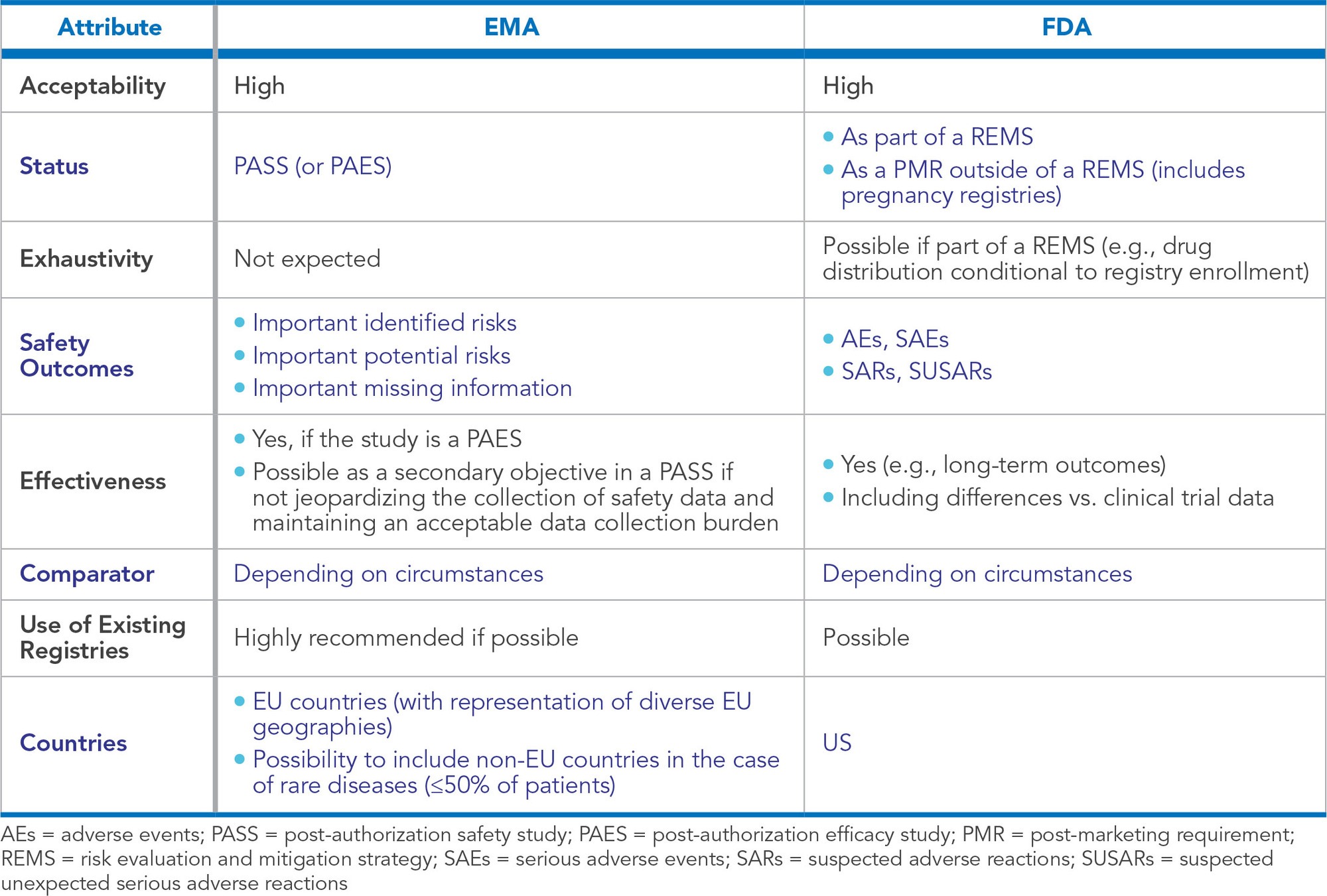
Acceptability of registries is high in all geographies. Figure 2 shows the European Medicines Agency (EMA) and United States Food and Drug Administration (FDA) positions on registries based on regulatory guidances4,5 and our experience. In other geographies, prospective cohort studies are generally well received and are sometimes a preferred approach or a requirement, especially in countries where electronic healthcare databases are not available (e.g., in India or in Mexico).6
What are the Key Registry Design Considerations for a Post-Marketing Safety Study?
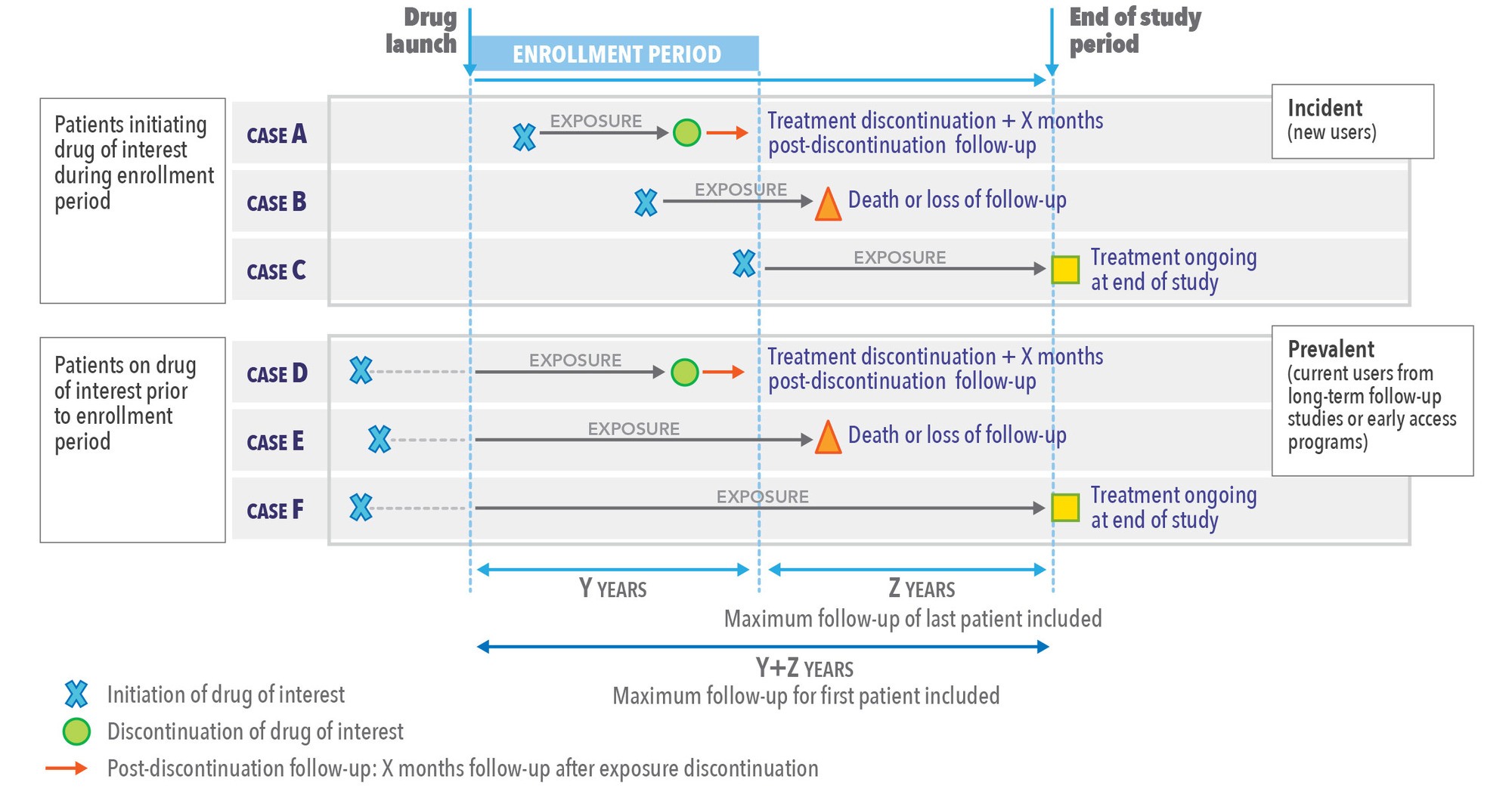
Inclusion Criteria: Incident Users or Prevalent Users?
Part of the objectives of a safety registry is to describe or confirm the risk function of one or several risks. For this reason, a key design point is the inclusion of new (or incident) users, in other words the inclusion of patients when they first initiate treatment with the drug of interest (or the comparator drug, if any). This is a key feature to avoid immortal time bias7 and depletion of susceptibles.8
However, in the case of rare disease, there is a need from both the market authorization holder (MAH) and regulatory authority perspective to collect as much data as possible. In this circumstance, it might be required that patients already treated outside of randomized controlled trials (e.g., through long-term safety follow-up studies or early access programs) who transition to routine treatment with the newly commercialized drug be included in the registry. These patients (prevalent users) already treated with the drug of interest for quite some time can increase the pool of longer-term drug exposure data. However, as such patients need to remain on treatment to qualify for study inclusion at the time of registry initiation, the resulting study population tends to be selected because those who died or discontinued the drug (e.g., due to AEs) prior to the enrollment into the registry are excluded.
The recommendation in that case is to ensure and clarify in the study synopsis that these patients will be analyzed separately and that there will be no pooling of the data from incident and prevalent users for the analysis of safety risks. Accordingly, the sample size calculation should be based on incident users only, and prevalent users should be included as additional participants (resulting in a greater overall sample size).
Follow-Up Duration
Fixed Follow-Up Duration or Fixed Study Duration?
Although it would seem quite straightforward to apply the same follow-up duration to all patients, this approach has the following caveats:
- The safety events of interest are observed in relation to the drug, so the period of interest is the exposure to the drug. The exposure duration is bound to vary from patient to patient and cannot be determined per protocol in a non-interventional study.
- Some patients might die or drop-out, thus truncating their follow-up.
- Proposing a fixed patient follow-up duration can delay the end of the study (e.g., if most of the patients have been included in the first half of the enrollment period and only a few patients have been included at the end of the enrollment period).
- Some safety events of interest may take several years to develop, even after exposure to the drug has ceased.
The recommended approach is usually to define an enrollment period and an end of study period which ensures an optimal minimal follow-up duration for the last patient included (See Figure 3). This approach allows accounting for the variable exposure period among patients; for censoring, which is common in any prospective follow-up; and, allows the assessment of the incidence rate of safety events (with exposed person-time at risk as the denominator), including long-term safety events of interest.
Follow-Up: How Long is Long-Term?
Registries are frequently aiming at assessing the long-term safety of drugs. But what does long-term mean? It depends, of course, on the expected length of treatment, the underlying pathogenic mechanisms and timing of occurrence of the safety events, and on the patient life expectancy related to the disease. Long-term safety will apply to chronic diseases and long-term treatments. Although no clear definition is given, a consensus is that long-term follow-up is usually five years or more.
Beyond Exposure: Should the Follow-Up Extend Post-Discontinuation of the Drug of Interest?
It is implicit in a post-marketing safety registry that the safety assessment will focus on the exposure period (period when the patient is exposed to the drug, from initiation to discontinuation). However, it is important to raise the question of the post-discontinuation follow-up, based on current level of knowledge on pharmacokinetics and other considerations (e.g., carcinogenicity, risk of withdrawal syndrome, potential reversibility of effect). Even when safety risks are expected to be very low or inexistent post-discontinuation based on these criteria, a short post-discontinuation follow-up period is usually well-received.
Practically, it is not always expected to pursue the same level of data collection in the post-discontinuation follow-up period: a simple remote point of contact with the site, patient, or family can provide enough information regarding the main safety events and vital status.
Figure 3 gives an example of study design based on a fixed study duration approach, including post-discontinuation follow-up.
Comparator or No Comparator?
The inclusion of a comparator group in a post-marketing safety registry is an important strategic decision. When possible and relevant, including such a group is often favored and requested by the EMA and FDA (although more seldomly in Asia and Latin America).
The relevance of adding a comparator group depends on several factors, such as:
- Presence of a clear standard of care on the market (one or several drugs) in the same indication, involving the same monitoring procedures
- Results of any head-to-head clinical trials
- One of the safety events of interest could be due to a drug class effect
In situations where a comparative study design is not possible or not relevant (e.g., drug of interest is first-in-class or first-in-indication), it can be useful to benchmark the incidence of the safety events of interest against the background risks (e.g., given in European risk management plans, or calculated concomitantly to registry analyses based on other data sources).
How to Optimize a Post-Marketing Safety Registry Design Using Existing Disease Registries
Use of Existing Disease Registry
In some therapeutic areas (e.g., oncology or rare diseases), disease registries are established to better describe the natural history of the disease and its outcomes under current standard of care treatments. Most of the time, these registries are established at the initiative of academic clinical centers, sometimes with the collaboration of patient associations or advocacy groups. While post-marketing safety registries are usually product registries, it is important to consider upfront whether to collaborate with existing disease registries in the same therapeutic area and in the geographies of interest, or establish a new registry, for the following reasons:
- Post-marketing safety registries might compete for enrollment with disease registries (with a risk of patient under-enrollment and selection bias in the product registry)
- Post-marketing safety registries might compete for data collection with disease registries (e.g., a patient participating in a disease registry could also be eligible for a post-marketing safety registry, thus potentially duplicating the data collection burden for the site).
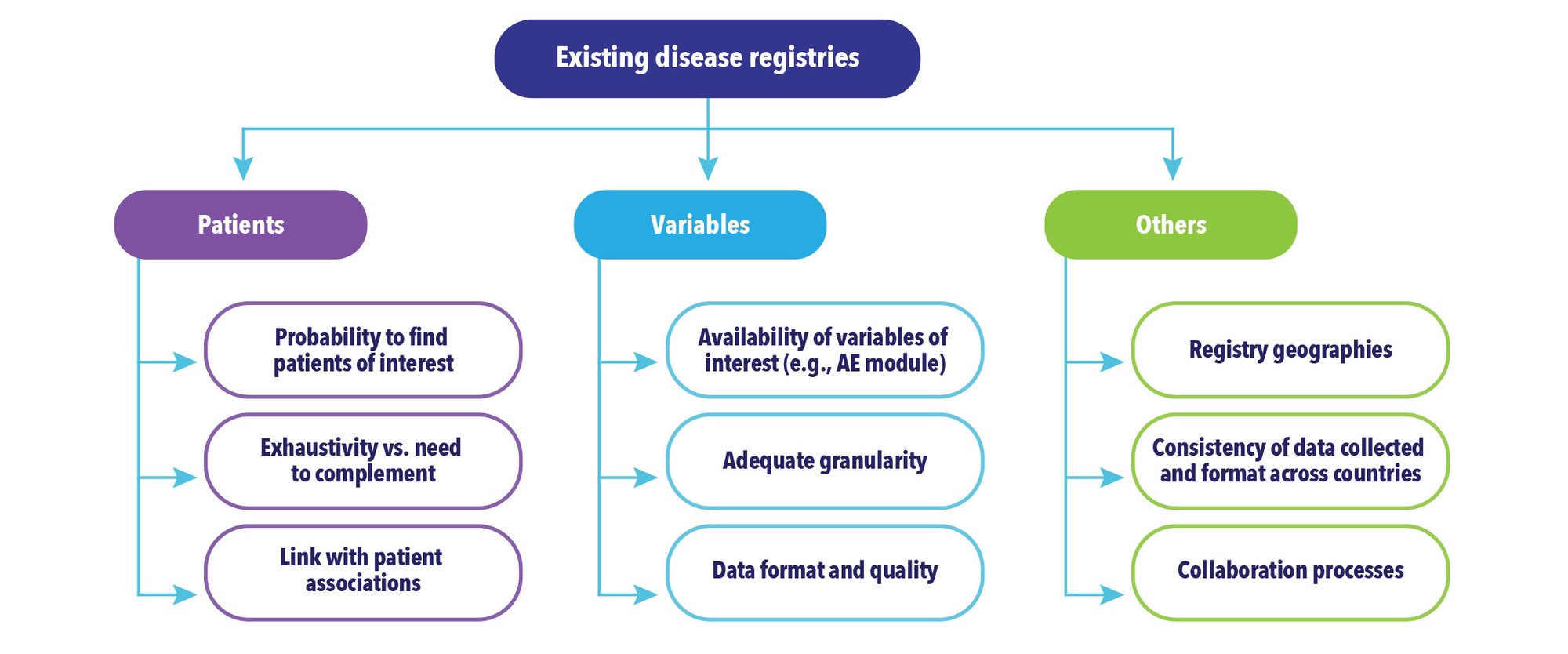
The EMA identified this situation as early as 2015 and has since suggested that existing registries could be used as a potential data source for post-marketing safety assessments, under certain conditions (e.g., quality requirements, collection of relevant data9). To this purpose, the patient registry initiative was launched and has yielded recent developments, such as conclusions of a workshop with different stakeholders, launch of a registry qualification process, and issuance of a draft guidance for public consultation.10-12 In our experience, the EMA is encouraging MAHs to explore the possibility of using existing registries and to collaborate with existing registries when possible. Figure 4 shows the main topics for discussion when considering collaborating with an existing registry.
Based on the analysis of these criteria, several strategies can be envisioned:
- No possibility to use existing registries (in this case, a strong justification should be given)
- Possibility of using the existing registry, but the registry needs to be complemented (e.g., with patients from other countries, by recruiting patients outside of the registry to achieve the targeted sample size, or with collection of additional key variables not collected in the registry)
- Possibility of using the existing registry as the only source of data
The best approach is to make early contacts with the registry owners to be able to evaluate any potential gap and to discuss any improvement steps (regarding geographical reach, data collection, EMA qualification) which would prove beneficial to both the MAH and the registry owner.
Building a Disease Registry to Optimize Future Post-Marketing Safety Studies
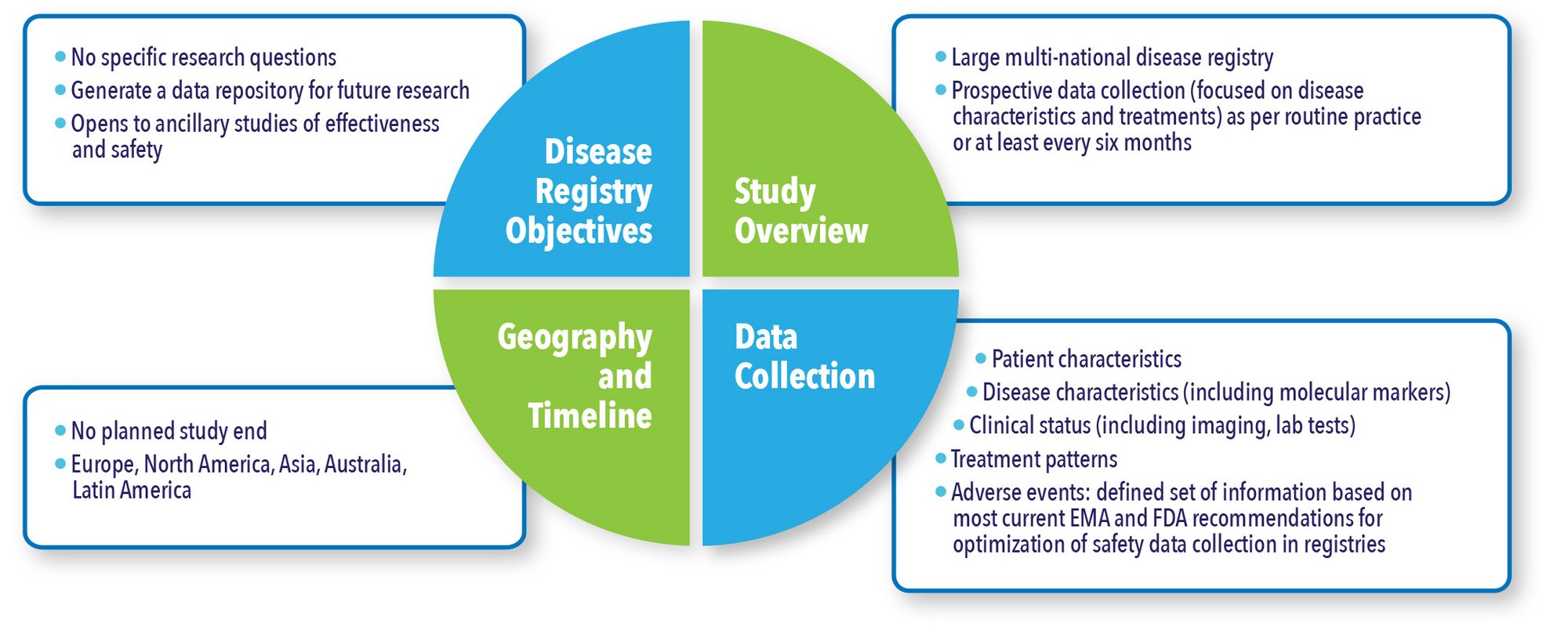
Conversely, when building a new disease registry, the possibility to use this registry as a data source for future post-marketing safety studies is ideally integrated from the outset in the study design (See Figure 5). Industry sponsors can indeed decide to invest in a disease registry in order to have a platform of data to leverage for different purposes, including safety assessment.
Figure 5. Case Study: Disease Registry Set-Up as a Platform for Future Research
Conclusion
Post-marketing safety registries, although thought to represent about 10% of post-marketing safety studies, will still be needed in several circumstances (e.g., rare diseases, high granularity of safety data, lack of existing electronic healthcare database). In these cases, beyond providing post-marketing safety data, these registries provide useful long-term information on treatment utilization and effectiveness outcomes that can be used outside of a regulatory safety environment.
The growing experience with this kind of safety registry (See Figure 6) allows the sponsor to anticipate and address the regulatory authorities’ requirements; it also ensures maximal leveraging of existing disease registries, and optimization of the study design to ensure the study objectives are achieved with the best balance between regulatory, scientific, and operational considerations.
References
- European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP) – Module VIII – Post-authorisation Safety Studies (Rev 3). 9 October 2017. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-viii-post-authorisation-safety-studies-rev-3_en.pdf. Accessed September 13, 2020.
- European Medicines Agency. The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) Guide on Methodological Standards in Pharmacoepidemiology EMA/95098/2010 Rev.7. Available at: http://www.encepp.eu/standards_and_guidances/documents/ENCePPGuideonMethStandardsinPE_Rev7.pdf. Accessed September 13, 2020.
- Pacurariu A, Plueschke K, Alonso Olmo C, Kurz X. Imposed Registries Within the European Postmarketing Surveillance System: Extended Analysis and Lessons Learned for Regulators. Pharmacoepidemiol Drug Saf. 2018 Jul;27(7):823-826. doi: 10.1002/pds.4449. Epub 2018 May 11.
- European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP) – Module VIII – Post-authorisation Safety Studies (Rev 3). 9 October 2017. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-viii-post-authorisation-safety-studies-rev-3_en.pdf. Accessed September 13, 2020.
- US Food and Drug Administration (FDA). Postmarketing Studies and Clinical Trials – Implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act Guidance for Industry – Draft Guidance. October 2019, Revision 1. Available at: https://www.fda.gov/media/131980/download. Accessed September 13, 2020.
- Haque A, Daniel S, Maxwell T, Boerstoel M. Postmarketing Surveillance Studies-An Industry Perspective on Changing Global Requirements and Implications. Clin Ther. 2017 Apr;39(4):675-685. doi: 10.1016/j.clinthera.2017.03.011. Epub 2017 Apr 7.
- Suissa S. Immortal Time Bias in Pharmaco-Epidemiology. Am J Epidemiol. 2008 Feb 15;167(4):492-9. doi: 10.1093/aje/kwm324. Epub 2007 Dec 3.
- Moride Y, Abenhaim L. Evidence of the Depletion of Susceptibles Effect in Non-Experimental Pharmacoepidemiologic Research. J Clin Epidemiol. 1994 Jul;47(7):731-7. doi: 10.1016/0895-4356(94)90170-8.
- McGettigan P, Alonso Olmo C, Plueschke K et al. Patient Registries: An Underused Resource for Medicines Evaluation: Operational Proposals for Increasing the Use of Patient Registries in Regulatory Assessments. Drug Saf. 2019 Nov;42(11):1343-1351. doi: 10.1007/s40264-019-00848-9.
- European Medicines Agency. Inspections, Human Medicines, Pharmacovigilance and Committees Division. Report of the workshop on the use of registries in the monitoring of cancer therapies based on tumours’ genetic and molecular features – 29 November 2019. Patient registries initiative. 27 March 2020; EMA/661159/2019. Available at: https://www.ema.europa.eu/en/documents/report/report-workshop-use-registries-monitoring-cancer-therapies-based-tumours-genetic-molecular-features_en.pdf. Accessed September 13, 2020.
- European Medicines Agency. Product Development and Scientific Support Department. Qualification Opinion on The European Cystic Fibrosis Society Patient Registry (ECFSPR) and CF Pharmaco-epidemiology Studies. 28 September 2018; EMA/CHMP/SAWP/622564/2018. Available at: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/qualification-opinion-european-cystic-fibrosis-society-patient-registry-ecfspr-cf-pharmaco_en.pdf. Accessed September 13, 2020.
- European Medicines Agency. Guideline on registry-based studies – Draft. 24 September 2020. EMA/502388/2020. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-registry-based-studies_en.pdf. Accessed: September 30, 2020.
For more information, please contact
[email protected], [email protected], or [email protected].